1. Introduction
Chronic constipation is a common and costly complaint. More than 4 million patients per year complain of frequent constipation [1]. The interstital cells of Cajal (ICC), known to generate the slow wave activity and to be involved in intestinal neurotransmission and the enteric nervous system (ENS), are suspected to play an important role in normal intestinal motility. Their role as intestinal pacemakers has been reported in many studies. ICC in vitro produce electrical slow wave and are the source of smooth muscle rhythmic electrical activity in the normal intestine [2-4]. Electrical slow wave are not observed in mice lacking ICC networks which demonstrate the absent or delayed intestinal motility [5,6]. ICC are also found to be diminished or lost in human disease with associated alterations in gastric and small intestinal motility, such as diabetic gastroparesis [7], intestinal pseudo-obstruction [8,9], and congenital absence of the enteric nervous system etc. [10]
Rhubarb (Da Huang) is one of the oldest and bestknown Chinese herbal medicines, first recorded in the Classic of the Materia Medica [11], Shen Nong Ben Cao Jing of the Han dynasty, and is classified as a top medicinal plant [12]. As described in Pharmacopoeia of People’s Republic of China, the most commonly used species are Rheum palmatum L., Rheum tanguticum’ Maxim. Ex Balf. Or Rheum officinale Baill., and are widely used in clinic to relieve constipation by purgation [13]. However, long term administration of rhubarb will cause slow transit constipation (STC) and secondary constipation after discontinuation. It was reported that long term use of rhubarb could have impairment on ICC which probably was the important reason for gastro-intestinal disorder [14-16].
Emodin (Figure 1) is the main active ingredient of rhubarb (2.6%), and is also used in the treatment of constipation. In the early investigation, our lab found that emodin could antagonize the toxic effect of aconitine on colonic ICC [17]. But in the same time, we also found emodin had toxicity effect on colon ICC. The results showed that emodin had efficacy or toxicity on ICC according to different dosage. In the study, we further investigate the toxicity mechanism of emodin on ICC function to explain the toxicity of rhubarb, uncover the substantial base of digestion disorder caused by rhubarb, and provide the reference for clinical safety dosage.
2. Materials and Methods
2.1. Drugs and Animals
Emodin was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). PBS (Lanzhou National Hyclone Bio-Engineering Co., Ltd., China); M199-culture medium, Collagenase type II, Ficoll 400 and MTT (Sigma Co., Ltd., USA); acetone (Cheng du Kelong solution Co., Ltd.), goat serum (Zhongshan Goldenbridge Biotechnology Co., Ltd), c-kit mAb (Wuhan Boster Biological Technology, Ltd.), anti-Rabbit IgG (Beijing Biosynthesis Biotechnology Co., Ltd.), all kits (Nanjing Jiancheng Bioengineering Research Institute).
The KM neonate mice (10 - 15 days old) were supplied by the Animal Experiment Center of Chengdu University of Traditional Chinese Medicine. All animal studies were performed according to the Guidelines for the Care and Use of Laboratory Animals that was approved by the Committee of Ethics of Animal Experimentation of Chengdu University of Traditional Chinese Medicine.
Emodin powder was weighted precisely and dissolved with 1 mol/L NaOH (pH was adjusted to 7.0 using M199). The emodin solutions were prepared before experiment.
2.2. Cell Isolation and Identification
The isolation of colon ICC cell was established in our laboratory [17] with some modifications of the former

Figure 1. The chemical structure of emodin.
reports [18,19]. Colons of KM mices (10 - 15 d) were isolated and washed twice with ice-cold PBS solution. After removal of serosa, mucosa and blood vessels, colons were digested in PBS containing 1.3 mg/mL collagenase type II at 37˚C for 10 min, and the cell suspension was collected. This process was repeated three times. Then cell suspensions were centrifuged at 4˚C and 1500 rpm for 3 min, and the pellet was resuspended in 10 mL Medium 199, and filtrated. 5 mL cells filtrate were added into centrifugal tube with 5 mL Ficoll 400, and then were centrifuged at 500 rpm for 10 min. The cells sediments in the middle of two fluid levels were collected and diluted to 1 × 105 cell/mL with Medium 199 (supplemented with 20% NBS, 50 μl/mL antibiotic-antimycotic solution and 10 ng/mL recombinant Murine SCF). Cells were plated on 24-well culture plates and maintained at 37˚C, in 5% CO2 incubator. After 24 h, a confluent monolayer of slowly synchronously ICC cell was developed. The medium was replenished every 2 - 3 d. Figures 2(a)-(c) showed the cell morphology of ICC in logarithmic growth phase.
ICC cells were identified after being cultured for 12 days. Cells were washed 3 times with PBS solution, and then fixed with acetone (1:1 dilution with PBS.) for 10 min. After cells were blocked with goat serum for 30 min, c-kit mAb (1:100 dilution) were added at 4˚C to stay overnight. FITC-labeled Anti-Rabbit IgG (1:50 dilution) was added to stay 1 h in dark at normal temperature. Finally the cells were washed three times with PBS solution, and observed under fluorescence microscope (Leica) after 150 µl PBS was added to each hole (Figure 3).
2.3. Measurement of Minimum Toxicity Concentration and Critical Time of Emodin on Colonic ICC
To investigate the minimum toxicity concentration of emodin on the function of colonic ICC, uniform design was used to design the study. Two factors (concentration and action time) and 7 levels for each factor were set up based on uniform design table U7 (74). After the cells were equilibrated with M199 for 24 h, emodin of different concentrations were added and remained for the scheduled time respectively. Cell viability as an index of drug toxicity was measured by MTT assay. Water-soluble MTT entered the cells by passive and/or active mechanisms, and subsequently it was reduced to its waterinsoluble formazan product by flavin oxidase [20]. In brief, following the partial removal of culture medium, cells were incubated with MTT (5 mg/mL) for 4 h at 37˚C. Then the medium was removed, and cells were oscillated in 150 µl DMSO for 15 min using the decolorization shaker. Finally, the optical density (OD, Table 1) was measured at 490 nm using the automatic enzymelinked immunosorbent assay systems (Thermo Co., Ltd.).
 (a)
(a)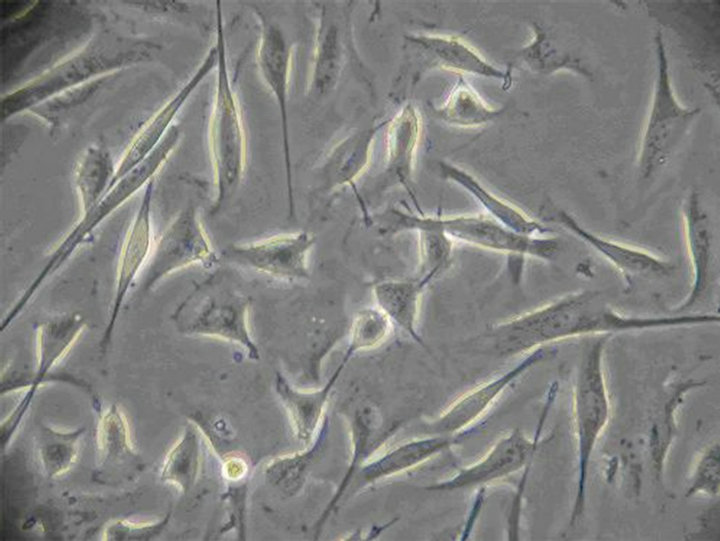 (b)
(b)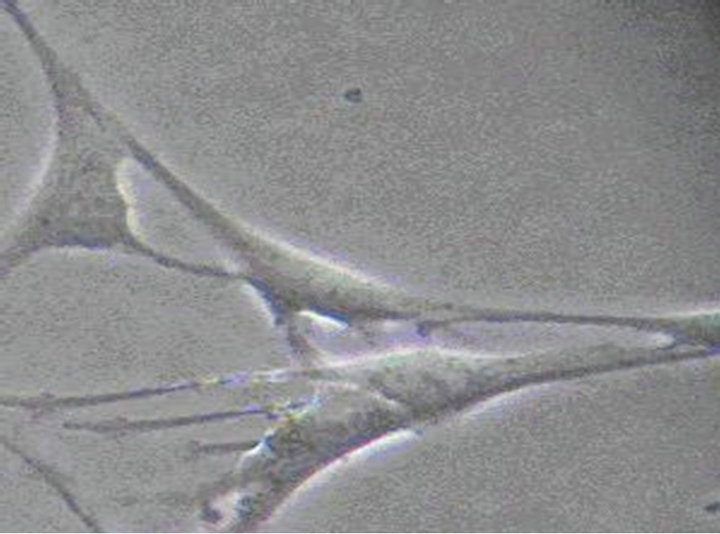 (c)
(c)
Figure 2. (a) The 12nd day of ICC (×100) The irregular shaped cells connected together via ecphyma, the formation of network structure; (b) The 10th day of ICC (×200) ICC are spindle-shaped, triangular. They connected neighboring cells via ecphyma; (c) The 12nd day of ICC (×400) ICC have the large nuclear and a small amount of perinuclear cytoplasm.
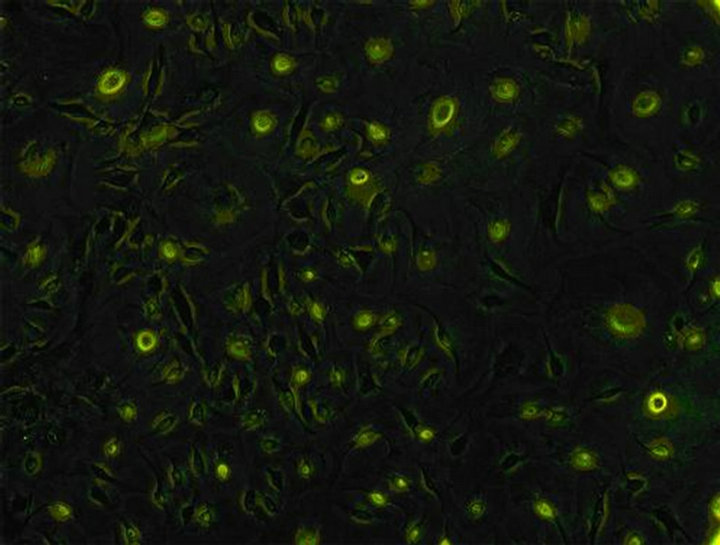
Figure 3. Identification of ICC (×100) the c-kit of ICC’s surface was positive. Both the cell bodies and ecphyma showed specific green. Cells linked up with each other, showing a large amount of ICC cells.

Table 1. OD value of emodin in 490 nm with different concentration and time ( ± SD, n = 8).
± SD, n = 8).
To authenticate the minimum toxicity concentration of emodin, a serial of concentrations were set up (0.005%, 0.001%, 0.0008% and 0.0005%). After equilibrating the cells with M199 for 24 h, emodin of different concentrations were respectively added and remained for 30 s. The OD measurement was carried out as described above (Table 2).
To investigate the critical time of emodin of minimum toxicity concentration on function of ICC, emodin solution (0.001%) were added to each well and reaction time were set as 0.5 min, 1 min, 15 min, 30 min, 60 min, 120 min, and 240 min. (control group were added 200 µl M199 without serum). The OD measurement was carried out as described above (Table 3).
2.4. Toxic Effect of Emodin on Membrane, Electrolytes, ATPs, Metabolism and Second Messenger
Drug administration: On the 12th day, after the cells were equilibrated with M199 (without NBS, SCF and antibiotic-antimycotic solution) for 24 h, 0.001% emodin were added to each well (control group were added 200 µl M199 without serum) and reaction time were set as 0.5, 1, 30 and 60 min respectively. After emodin was removed, the cells were treated as following.
To measure the concentration of MDA and LDH, M199 (containing 20% NBS) was added to each well. And the cells were cultivated in the CO2 incubator for an hour. 200 or 500 μl supernatant of each well was taken to measure the MDA or LDH concentration with MDA or LDH kit.
To measure the concentration of protein, ACP, Na+, Ca2+ ,Na+-K+-ATPase and Ca2+-ATPase, the cells were digested for 5 min with 100 μl collagenase II, and then 200 μl PBS were added. Cell suspension was collected to be centrifuged at 1000 rpm for 10 min. And then, the cell was washed and resuspended twice with PBS and the supernatant was abandoned. Deionized water was added

Table 2. OD value of emodin in 490 nm with different concentration and time ( ± SD, n = 8).
± SD, n = 8).
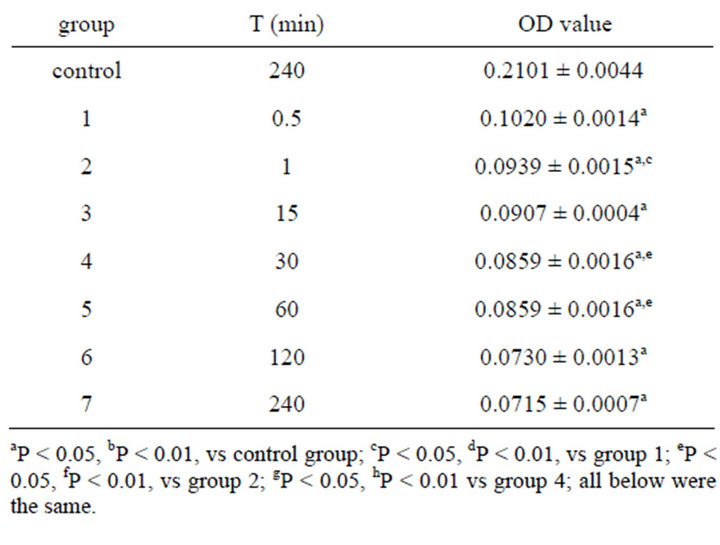
Table 3. Effect on OD value of 0.001% emodin in 490 nm ( ± SD, n = 8).
± SD, n = 8).
to make suspension well-distributed and cells were smashed by homogenizer. Protein concentration was measured by Coomassie brilliant blue (CBB) kit and ACP concentration was measured with ACP kit. K+ concentration was measured with K+ kit by the method of protein hydrolysis enzyme. Na+ concentration with Na+ kit were measured by turbidimetry. Ca2+ concentration with Ca2+ kit were measured by colorimetry. Na+-K+- ATPase and Ca2+-ATPase concentration were measured with ATP kit.
The ICC metabolism was observed in the same way as the electrolyte, and glycogen concentration was measured with glycogen kit.
To measure the concentration of IP3 and cAMP, the cells were digested for 5 min after 100 μl collagenaseⅡ was added, and then 200 μl PBS was added. After cells were dropped off, cell suspension was collected to be centrifuged at 1500 rpm for 5 min. The density of the cell was adjusted to 106/mL after being resuspended in PBS. Cells were broken and components inside them were released through repeated freezing and thawing. Then the suspension was centrifuged at 3000 rpm for 20 min and the supernatant was collected to measure the IP3 and cAMP concentration with IP3 and cAMP kits.
2.5. Statistical Analysis
After the text edit has been completed, the paper is ready for the template. Duplicate the template file by using the Save as command, and use the naming convention prescribed by your journal for the name of your paper. In this newly created file, highlight all of the contents and import your prepared text file. You are now ready to style your paper.
3. Results
3.1. Cells Isolation and Indentification
New cultured cells were granule or clump, and most of them adhered to walls within 36 h. The cells were variable in shape (fusiform, triangular and stellate) from the second day of adherence. The division growth speeded up, and became confertim ellipse or stellate cell which was single layer or multi layers at the 12nd day. The irregular shaped cells connected together via ecphyma (Figure 2). These cells, labeled with an antibody to c-kit protein, formed a dense wet, showed specific green under Leica automatic microscope (Figure 3).
3.2. Minimum Toxicity Concentration and Critical time of Emodin on Colonic ICC
Table 1 showed the OD value after the cell was treated with emodin of different concentrations at different time. And the data were analyzed by uniform design software 3.0 (China). The equation for emodin analyzed by uniform design software 3.0 was: y = 0.132 − 0.0716X1 − 0.0000523X2 + 0.00011X1X2 (X1—concentration of emodin, X2—reaction time). The result was significant: α = 0.05, S = 6.41e−3, SS = 4.11e−5, Ft = 17.555, R = 0.9727. And the results showed that the minimum toxicity concentration of emodin was 0.001%, and the critical time of toxic effect was 30 s after administration. No significant difference was found between the control group and the group treated with emodin of concentration below 0.001% (Table 2). The result verified the conclusion that 0.001% was the minimum toxicity concentration of emodin on colonic ICC.