Radioiodination and biological evaluation of valsartan as a tracer for cardiovascular disorder detection ()
1. INTRODUCTION
Myocardial assessment continues to be an issue in patients with coronary artery disease and left ventricular dysfunction. Nuclear imaging has long played an important role in this field. In particular, Positron emission tomography (PET) imaging using 18F-fluorodeoxyglucose is regarded as the metabolic gold standard of tissue viability [1,2], which has been supported by a wide clinical experience. Viability assessment using single photon emission computed tomography (SPECT) techniques has gained more wide-spread clinical acceptance than PET, because it is more widely available at lower cost. Moreover, technical advances in SPECT technology such as gated-SPECT further improve the diagnostic accuracy of the test. Further efforts are necessary to improve the diagnostic performance of nuclear imaging as reliable viability test [3].
Advances in imaging methodology aim at more rapid, more accurate acquisition and hybrid systems additionally, the advent of various molecular-targeted probes is expected to result in novel clinical applications of nuclear cardiology in the future. Several radiopharmaceuticals have been introduced for the scintigraphic visualization of myocardium and cardiovascular disorders. So Valsartan was chosen as a compound for radio-iodination with the radioisotope iodine-125.
Valsartan (Angiotan) is an angiotensin II receptor antagonist (more commonly called an “ARB”, or angiotensin receptor blocker), with particularly high affinity for the type I (AT1) angiotensin receptor. By blocking the action of angiotensin the peripheral blood vessels are allowed to widen, which means that there is more space and less resistance in these blood vessels. This is the main mechanism by which the pressure in the blood vessels is lowered [4,5]. Blocking the actions of angiotensin II also increases the amount of fluid removed from the blood by the kidneys. This decreases the amount of fluid in the blood vessels, which also lessens the resistance and pressure in the blood vessels. The combined effect of these changes is to lower the blood pressure, hence valsartan is used to treat high blood pressure [6,7]. The reduced pressure within the blood vessels also means that the heart doesn’t have to work as hard to pump the blood around the body. Studying of valsartan Pharmacokinetics, disposition and biotransformation was done using [14C]-radiolabeled valsartan in healthy male volunteers after a single oral dose [8].
The aim of this study is to investigate all factors affecting the labeling process and optimize the conditions required to get a labeled compound with high radiochemical yield and purity. Also, the biodistribution and the efficiency of labeled valsartan in heart and blood vessels will be experimentally evaluated in normal animals. During this study, 125I was used in the labeling process due to its availability, suitable energy and half-life for research.
The suggested structure of 125I-Val was formed via an electrophilic substitution reaction in the presence of iodogen as oxidizing agent as shown in Figure 1.
2. EXPERIMENTAL
2.1. Materials and Methods
All chemicals used in the present work were of analytical grade. Valsartan, was obtained from Global Naby Pharmaceutical Company, Egypt and was used without any purification. Absolute ethanol was used as a solvent. Double distilled water was used for all experiments. Chloramine-T [N-chloro-P-toluene sulfonamide salt (CAT)] from Aldrich and iodogen (1,3,4,6-tetrachloro- 3a,6a-diphenyl glycoluril) from Pierce Chemical Company. Thin layer chromatography (TLC) aluminum sheets (20 × 25 cm) SG-60 F254 (Merck). Na125I (185 MBq/5 mL) in diluted NaOH, pH 7-11 was purchased from institute of isotopes, Budapest, Hungary.
2.2. Equipment
Radioactivity was measured by means of a gamma counter (Nucleous Model 2010) connected with a well type NaI (Tl) crystal. High Performance Liquid Chromatography (HPLC) [Sykam Model], S 2100 Solvent delivery system, S 5111 injector valve bracket, S 3240 UV/ VIS spectrophotometric detector and Lichrosorb analytical column C-18 (250 mm × 4 - 6 mm, 5 mm). Electrophoresis was performed with an EC-3000 P-series programmable device (EC Apparatus Corporation) and chamber supply units using cellulose acetate strips.
2.3. Animals
Swiss Albino mice weighing 30 - 35 g were purchased from the agriculture research center, Cairo, Egypt. The animals were kept at constant environmental and nutriational conditions throughout the experimental period and kept at room temperature (22˚C ± 2˚C) with a 12 h on/off light schedule. The food and water were standard.
2.4. Labeling of Valsartan with Na125I Using Iodogen as Oxidizing Agent
An appropriate volume of iodogen solution added to

Figure 1. The suggested structure of 125I-Val.
clean glass culture tubes and dried under nitrogen atmosphere. Iodogen was deposited on the wall of the glass tubes as a thin film. The tubes were then closed and stored at 4˚C till use. Iodination reactions were directly carried out in these tubes. A suitable concentration of valsartan was placed. For labeling, 5 µl of Na125I (3.7 MBq) was added, and then, the reaction mixture was kept at different temperature for interval times. The reaction was stopped simply by removing the aqueous phase and also quenched by the addition of sodium metabisulphite to assure complete cessation of the oxidative process [9].
2.5. Radiochemical Analysis
The radiochemical yield of 125I-Val was determined by TLC & electrophoresis and the radiochemical purity was determined using HPLC.
2.6. Thin Layer Chromatography (TLC)
The radiochemical yield % of 125I-Val was determined using aluminum-backed silica gel 60. A volume of 5 µl reaction mixture was placed on the start line, then chromatographed using methylene chloride: ethyl acetate (2:1 v/v) [10] as a developing system. The strips were removed, dried and cut into 1 cm segments and assayed for radioactivity using SR.7 gamma counter. The relative first Rf for free 125I = 0.0 − 0.1 and Rf for labeled compounds = 0.9. The radiochemical yield was calculated as the percent ratio of activity of labeled compound relative to the total activity on the TLC-strip.
2.7. Electrophoresis
Electrophoresis was done using cellulose acetate strips. These strips were moistened with 0.02 M phosphate buffer pH 7 and then were placed in the chamber. Samples of 5 µL were applied at a distance of 12 cm from cathode. Standing time and applied voltage were continued for 90 min, developed strips were removed, dried and cut into 1 cm segments. They were counted using a well-type NaI(Tl) detector connected with a single-channel counter. An analysis of samples from the reaction mixture resulted in two peaks as shown in Figure 2, one corresponding to the free iodide which moved towards the anode with 11 cm distance while 125I-Val remained at the point of spotting, depending on their charge and ionic mobility [11]. It gave RCY equal to 98.6%.
2.8. HPLC
The detection of 125I-Val with high performance liquid chromatography was done using 0.2 M Sodium dihydrogen phosphate: Acetonitril with ratio (58:42), and the flow rate was 1 ml/min. As shown in Figure 8, two peaks were obtained. The first peak was at 2 minutes, whereas the second peak was at 7 minutes retention time. The first peak corresponds to free iodide, whereas the second peak corresponds to the 125I-Val as shown in Figure 3.
2.9. Biodistribution Studies
Albino mice were used for quantitative biodistribution studies. 200 µl (3.5 MBq) of 125I-Val was injected intravenously (i.v.) in the mice tail vein. Groups of three mice were used for each experiment. The mice were sacrificed by cervical dislocation at 0.5, 1 and 3 h after injection of the tracer, Blood samples were collected at the time of scarification. The different organs were removed, washed, weighted and counted then compared to the standard solution of the labeled valsartan. The average percent values of the administrated injected dose per organ (ID/ organ) were calculated. Blood, bone, and muscles were assumed to be 7%, 10%, and 40% of the total body weight, respectively [12].

Figure 2. Electrophoresis pattern of 125I-Val.
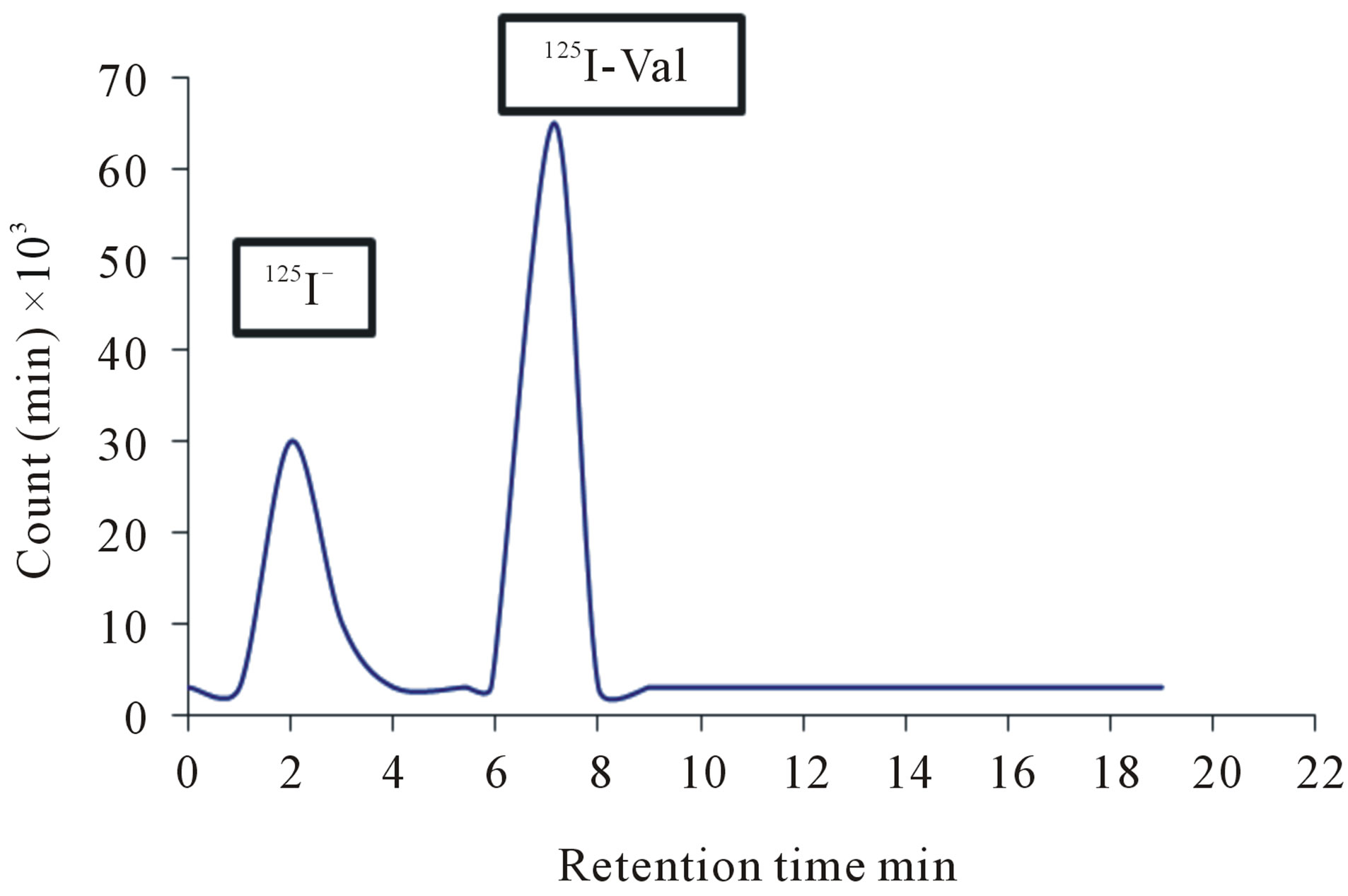
Figure 3. The HPLC separation of the labeled 125I-Val using 50 µg valsartan (0.2 mM) and 25 µg Iodogen (0.15 mM) in room temperature within 30 min.
3. RESULTS AND DISCUSSION
3.1. Effect of Valsartan Amount
The influence of different valsartan amounts on the percentage of RCY of 125I-Val using 50 µg CAT (0.439 mM) as oxidizing agent at room temperature within 30 min was investigated in Figure 4. The data indicated that valsartan amount has no significant effect on the RCY of 125I-Val. Maximum RCY of 125I-Val (91.9) was achieved with valsartan amount equal to 50 µg (0.2 mM). The RCY was not affected by increasing the amount of valsartan higher than (50 µg) (0.2 mM). This may be attributed to the fact that the yield reaches the saturation value because the entire generated iodonium ions in the reaction were captured at that concentration of valsartan [10].
3.2. Effect of Different Oxidizing Agents Amount
To achieve an electrophilic substitution reaction, a suitable oxidizing agent, which required to give the maximum RCY was selected by using different oxidizing agents such as chloramine-T (CAT), the sodium salt of nchloro-4-toluenesulfonamide, Iodogen (1,3,4,6-tetrachloro- 3α,6α-diphenylglycouril) and N-bromosuccinamide.
The influence of different CAT amounts on the percentage of RCY of 125I-Val using 50 µg valsartan (0.2 mM) at room temperature within 30 min was investigated in Figure 4. Maximum RCY of 125I-Val (91.9%) was achieved with CAT amount equal to 50 µg (0.439 mM) The RCY was decreased by increasing the amount of CAT. This is due to the fact that the high concentration of CAT causes a number of undesirable oxidative side reactions including chlorination [13] and polymerization of substrate [14].
The RCY of 125I-Val increased up to 91.9% using 25 µg Iodogen (0.15 mM), and this may due to negligible of the side reactions [9], and by increasing iodogen amount up to 300 µg (0.62 mM), the RCY decreases down to 27%. This attributed to precipitation of iodogen on the wall of the reaction vial with large amounts and the reaction total volume is small.
The RCY of 125I-Val decreases down to be 55% when N-bromosuccinamide used as oxidizing agent and this may due to the side reactions (Figure 5).
3.3. Effect of pH of the Reaction Mixture
Figure 6 shows the variation of the RCY of 125I-Val as a function of pH of the reaction medium in presence of iodogen as oxidizing agent. The data clearly showed that a high RCY was obtained at pH 7. When the pH of the reaction medium was shifted towards the acidic region, the yield decreased to 48.5% at pH 2, this may be attrib-