Catalytic Synthesis of Salicylate Esters over Cordierite Honeycomb Coated with Mo (VI)/ZrO2 ()
1. Introduction
Metal oxide based solid acids as heterogeneous catalysts are extremely useful in many large-volume applications, especially in the production of fine and specialty chemicals as well as in the petrochemical industry [1,2]. Among metal oxides, zirconia (ZrO2) is a versatile oxide used as a catalyst as well as a catalyst support and promisingly has been employed in many industrially important organic transformations. Zirconia based solid acids such as VOx/ZrO2, MoOx/ZrO2 & WOx/ZrO2 have been synthesized and used as catalysts in a few liquid & vapor phase reactions [3-5]. Though these zirconia based solid acid are superior catalysts and free form deactivation, they are less explored for organic synthesis [6].
Cordierite honeycomb monoliths as catalyst carriers have been used extensively in auto exhaust catalysis for gas phase reactions [7,8] but least explored in liquid or vapor phase organic reactions. Hence, honeycomb monolith coated with solid acids such as zirconia and its modified forms would be a novel and extremely useful technique in liquid phase reactions for the production of fine and specialty chemicals as well as in the petrochemical industry.
In the present article, work done on the synthesis of honeycomb coated with solid acids such as ZrO2 & Mo (VI)/ZrO2, their physico-chemical characterization and catalytic evaluation in liquid phase transesterification of methyl salicylate with various alcohols to synthesize their respective salicylate esters is described. Salicylate esters are industrially important and are mainly used in sun-tan lotions, antiseptic creams, etc.
2. Experimental
Zirconia (ZrO2) and Mo (VI)/ZrO2 (MZ) with 6% Mo (VI) were coated on honeycomb (HC) monoliths by impregnation method [8]. Typically, to coat 6% MZ on a HC, a dilute solution containing 5.4 g of zirconyl nitrate [ZrO(NO3)3∙8H2O] & 0.1 g of ammonium molybdate [(NH4)6Mo7O24∙4H2O] dissolved in 50 mL of deionised water was coated on a wash coated honeycomb by dipping and drying in a furnace pre-heated at 400˚C. The dipping and drying steps were repeated 8 to 10 times till ~0.2 g of 6% MZ is coated on the HC. The HC coated with 6% MZ was calcined at 550˚C for 5 h in a muffle furnace before its use as a catalyst. ZrO2 and 6% MZ were also prepared in powder form by impregnation method.
Thus prepared catalysts (in both HC coated and powder forms) were analyzed for their total surface acidity by NH3-TPD, crystallinity by powder XRD and morphology by SEM techniques.
The catalytic activity of all the catalysts used in the present study was determined in liquid phase transesterification of methyl salicylate (MS) with alcohols such as ethanol, iso-propanol, iso-butanol, iso-pentanol, cyclohexanol & benzyl alcohol to synthesize their respective salicylate esters. The transesterification was carried out in a batch reactor.
3. Results and Discussion
The total surface acidity (TSA) values of ZrO2 and 6% MZ (both in HC coated and powder forms) are given in Table 1. Zirconia was found to be less acidic when compared to 6% MZ indicating that the incorporation of Mo (VI) ions increases the number of acid sites on zirconia thereby increasing the TSA values of 6% MZ [9].
The PXRD patterns of the HC coated with zirconia consisted of peaks corresponding to both tetragonal (T) and monoclinic (M) phases (Figure 1). However, PXRD patterns of 6% MZ consisted of only peaks corresponding to tetragonal phase and the peaks corresponding to monoclinic phase are absent. The PXRD patterns of ZrO2 and 6% MZ in powder form were found to be similar to that of the PXRD patterns of their HC coated forms.
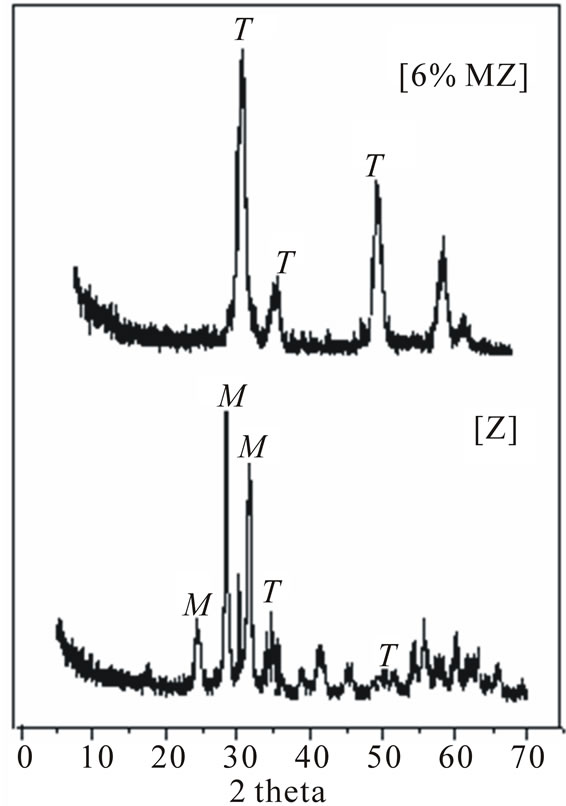
Figure 1. PXRD patterns of ZrO2[Z] & 6% MZ.
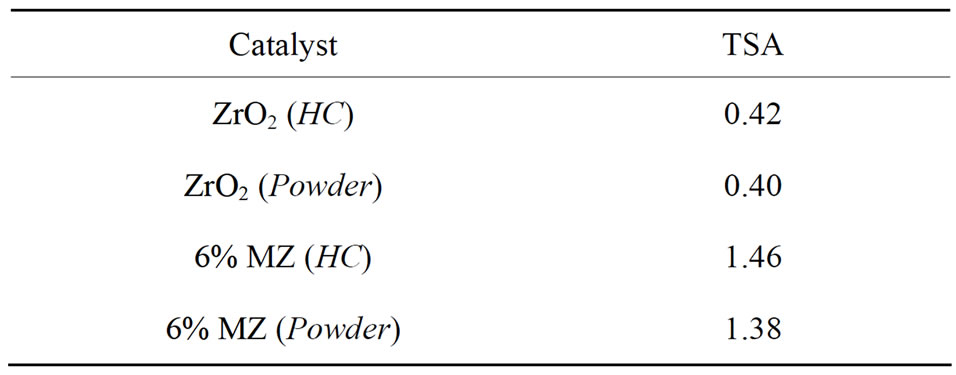
Table 1. Total surface acidity (TSA in mmols/g) values of catalysts.
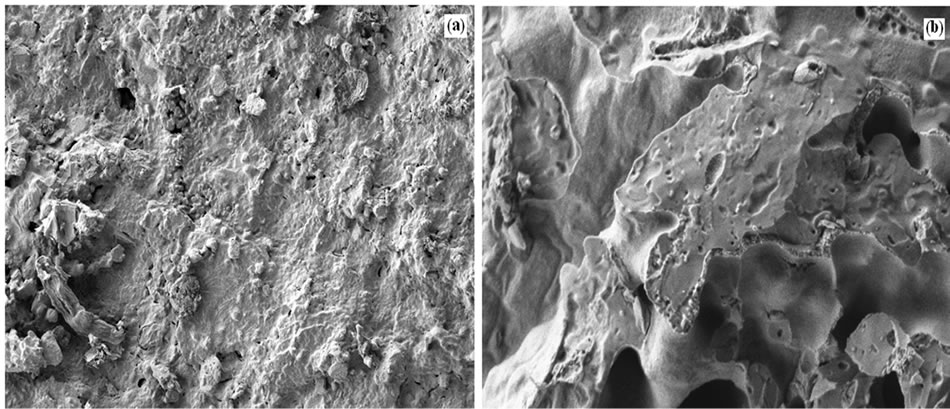
The information obtained from SEM images (Figure 2) of 6% MZ i.e., structures sticking to the surface of the HC indicated that the method used for coating the catalytic material on the honeycomb was suitable enough to obtain adherent coating of the catalytic material (MZ).
Transesterification of methyl salicylate (MS) with different alcohols (R) was carried out by taking MS: R ratio of 2:1 and the results are presented in Table 2. Since transesterification is a reversible reaction, excess of MS favors forward reaction leading to the formation of the desired product.
A correlation between the total surface acidity, PXRD phase and the catalytic activity of the catalyst was observed. 6% MZ which possessed higher total surface acidity and also consisted of catalytically more active tetragonal phase [10] showed higher yield of salicylate esters when compared to ZrO2 catalyst (Table 2). Both ZrO2 and 6% MZ were 100% selective towards the formation of salicylate esters.
Among different alcohols, since i-propanol and cyclohexanol are secondary alcohols they showed very less
Mag=1.00kx Auriga39-22 10um
Mag=20.00kx Auriga39-22 200um
Figure 2 Lower magnification; (b) Higher magnification.

Table 2. Transesterification of methyl salicylate with different alcohols over ZrO2 and 6% MZ [Reaction conditions: Molar ratio of methyl salicylate: alcohol (R) = 2:1; reaction temperature = refluxing temperature; catalyst weight = ~0.2 g of ZrO2 or 6% MZ; reaction time = 4 h].
yield due to more steric hindrance compared to primary alcohols.
When the catalytic activity of 0.2 g 6% MZ (in powder form) and 6% MZ (HC coated) were compared, even though same amount of the catalyst (~0.2 g) was used in powder as well as in HC form, a ~2 fold increase in the yield of salicylate esters was observed over HC coated form of the catalyst. This can be attributed to the higher geometric surface area of the honeycomb catalyst [8]. Further, when the reusability of 6% MZ in these two forms was checked, it was observed that the rate of decrease in the catalytic activity in powder form of the catalyst was more compared to its HC coated form.
4. Conclusion
In general, the solid acids coated on cordierite honeycomb monoliths are very efficient, economical, eco-friendly (3E concept) and novel catalysts for organic synthesis. These honeycomb catalysts are easy to prepare, efficient, economical, easy to use, easily recoverable, easily regenerable and re-usable. Therefore, solid acids coated on honeycomb are more advantageous than in their powder form. Honeycomb monolith coated with Mo (VI)/ZrO2 can especially be an excellent catalyst for transesterification to synthesize industrially important salicylate esters.
5. Acknowledgements
The corresponding author is thankful to DST, New Delhi for funding. The authors are thankful to the authorities of University of Malaya for SEM analysis, Department of Chemistry, St. Josephs College, Bangalore for PXRD analysis and IITM, Chennai for NH3-TPD analysis.
NOTES