Clinical Study on Autosomal Dominant Polycystic Kidney Disease among South Indians ()
1. Introduction
The polycystic kidney diseases (PKD) are the most common hereditary nephropathies and are reported to result in end-stage renal disease (ESRD) by the age of 60 - 70 yr [1-4]. Polycystic kidney diseases are primarily characterized by the progressive development of renal multiple epithelial cysts in both the kidneys. Polycystic kidney disease is broadly divided into 2 forms, autosomal recessive polycystic kidney disease (ARPKD) and autosomal dominant polycystic kidney disease (ADPKD) [4-8]. The diseases are caused by mutation either in PKD1 (85%) or PKD2 (15%) and PKD3 (rare) genes [7-12].
Autosomal dominant polycystic kidney disease (ADPKD) occurs worldwide in all races. ADPKD disease is one of the most commonly inherited conditions in humans with an incidence of 1:400 to 1:1000 [1,7,13,14]. It is genetically heterogeneous with two genes being identified, PKD1 (16p13.3) and PKD2 (4q21) [11,15,16]. The proteins encoded by PKD1 and PKD2 are called polycystin 1 and polycystin 2 [10,17,18], which interact with each other in the primary cilia of renal epithelial cells and participate in complex signal transduction pathways, which might be involved in mechanic/chemosensory functions and have some role in cell proliferation and maturation [10,16]. Fibrocystin is defective in autosomal recessive PKD [5,6,19,20].
The common complications noted in polycystic kidney disease include hypertension, macroscopic hematuria, urinary tract infection, renal calculi, diabetes mellitus, renal osteodystrophy, cardiac valve abnormalities, anemia, hernia of the anterior abdominal wall and cerebral berry aneurysm [21].
The impact of lipid abnormalities on renal function have been evaluated in various studies [22-24]. Common abnormalities include an elevation of serum or plasma triglycerides (TGL), a decrease in the high-density lipoprotein (HDL) cholesterol, and elevation in low-density lipoprotein (LDL) cholesterol and marked oxidation of LDL cholesterol [22]. Hypertriglyceridemia is the most common plasma lipid abnormality in patients with renal failure [25,26]. Dyslipdemia is a common complication of progressive kidney disease which also contributes to high cardiovascular morbidity and mortality of renal disease patients [27].
The Polycystins have regulatory role in ion transport. The polycystic epithelial cells have been reported to show propensity to secrete solutes and fluid rather than absorbing them [28], which would be directed to the regulation of ion permeability and possibly the misstep that initiates cytogenesis in patients with autosomal dominant polycystic kidney disease (ADPKD) [29].
In patients with PKD, both genetic and biochemical studies have been conducted in most of the populations worldwide (Caucasians, Japanese, Argentines, Canadian, Egyptian, Chinese, Italian and United States) by different researchers [3,5,6,24-26,30]. However, till now no study is available among south Indian (Madurai) population. Hence, the present study is focused on the lipid profile and the level of certain elements like Sodium (Na), potassium, iron and calcium in patients with Autosomal dominant polycystic kidney disease among south Indian (Madurai) population.
2. Methodology
The study group comprised of hundred clinically proven autosomal dominant polycystic kidney disease (ADPKD) patients (Table 1) of both the sexes in the age group of 10 - 80 years taking treatment in Madurai Government Rajaji hospital and Kidney Transplantation and Research Centre, Madurai, Tamil Nadu, India Ethical clearance was obtained and approval was also obtained from the Institutional Bio-safety committee (IBSC) of Lady Doak College. The age and sex matched healthy subjects were also selected from the general population. The blood samples were collected in EDTA coated tubes from Government Rajaji hospital and Kidney Transplantation and Research centre, Madurai and plasma was separated immediately. The samples were stored at 4˚C.
2.1. Biochemical Analysis
The total cholesterol (T. Chol), triglycerides (TGL), high density lipoprotein (HDL), low density lipoprotein (LDL)
Table 1. Prevalence of ADPKD in male and female in the age group of 10 - 80 yr among South Indian population.

and very low density lipoprotein (VLDL) were analysed using commercially available kit (Span Diagnostic kit). The levels were measured with Semi Autoanalyzer (Erba, Chem 5X). Sodium, potassium, iron, calcium and iron levels were estimated using Flame Photometer (ELICO, CL 22D) and Atomic Absorption Spectroscopy (AAS) (ELICO, SL 173).
2.2. Statistical Analysis
The mean and standard deviation was calculated for ADPKD patients and the control subjects and significant difference between ADPKD patients and the control was calculated by using student t test.
3. Results
It was observed that both males (48%) and females (52%) are equally affected by ADPKD and that most of the patients fall under the age group of 30 - 50 years. The study also has revealed that 72% of the patients have high blood pressure (hypertension) (Table 1).
ADPKD patients were observed to have complications like hematuria (12%), renal calculi (10%), urinary tract infection (13%), diabetic nephropathy (17%), cardiovascular problems (21%), renal osteodystrophy (13%) and anemia (14%) (Table 2). The results also revealed that most of the patients have hypertension (72%), diabetes and cardiovascular problems (21%) and anaemia (14%). Hence, lipid profile and minerals like calcium, sodium, potassium and iron were studied (Tables 3 and 4).
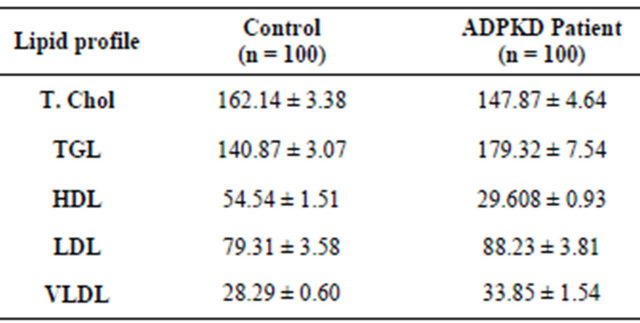
A significant increase in the level of triglycerides (TGL) (p < 0.001), low density lipoprotein (LDL) (p = 0.003) and very low density lipoprotein (VLDL) (p < 0.001) were observed in ADPKD patients when compared to the control subjects. There was very little difference in the amount of total cholesterol (T. Chol) (p < 0.05) between the patients and control subjects. The level of high density lipoprotein (HDL) was observed to be
Table 2. Analysis of ADPKD associated complications in ADPKD patients among South Indian (Madurai) population.
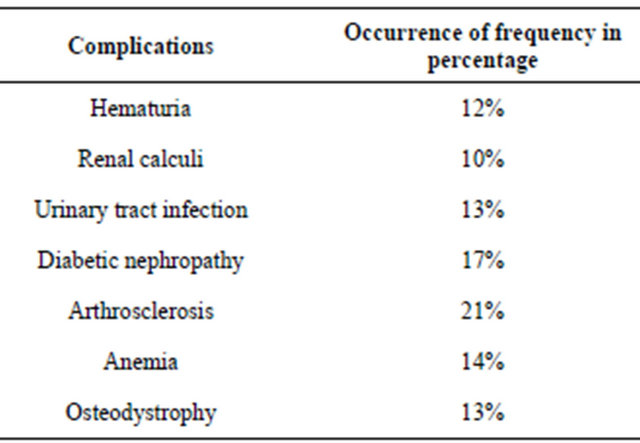
Table 3. Comparative analysis of lipid profile in control subjects and ADPKD subjects.
Values are mean ± standard deviation (mg/dl).
Table 4. Serum/plasma calcium (Ca), sodium (Na) and potassium (K) levels in control subjects and ADPKD patients.

Values are mean ± standard deviation (mEq/dl).
significantly decreased (p < 0.001) in ADPKD patients when compared to the control subjects (Table 3).
The level of calcium (Ca), sodium (Na), iron (Fe) and potassium (K) revealed the mean plasma calcium and potassium levels to be significantly high (p < 0.001) in ADPKD patients. The level of sodium and iron in ADPKD patients were significantly lower (p < 0.001) when compared to the control subjects (Table 4).
4. Discussion
In the current study, both male and female were observed to be equally affected by autosomal dominant polycystic kidney disease (ADPKD). This might be due to no sexual preponderance in the inheritance. Dyslipidemia, diabetes mellitus, hypertension and cardiovascular disease were also noted among ADPKD patients (Table 2). These complications might be due to low sodium, high calcium and increase in the level of triglycerides (TGL), total cholesterol, low density lipoprotein (LDL) and also decrease in the level of high density lipoprotein (HDL).
Dyslipidemia is reported to be a common complication of progressive kidney disease [27]. Several studies have shown hypercholesterdemia and hypertriglycerdemia to be the risk factors for renal failure [23]. In atherosclerosis, high TGL and low HDL levels have been shown to be associated with risk in developing renal dysfunction [31].
The results also indicate a significant increase in the level of TGL and LDL and significant decrease in the level of HDL in ADPKD patients when compared to the control subjects (Table 2). The total cholesterol level was also found to be normal in patients and it coincides with the results obtained by Dumm et al. [23,25,26]. They have also reported that Ischemic Heart Disease (IHD) and other associated complications of atherosclerosis are the most common causes of death in patients with renal failure (chronic renal failure) [25,26]. Diabetes and hypertension have been reported to be the leading causes for end-stage renal disease (ESRD) in the United States [24]. Trevisian et al. [24] have also stated that hyperlipidemia contributes not only to cardiovascular disease but also to renal progression.
Hypertension has been reported in 50% - 75% patients prior to renal insufficiency and is said to be responsible for accelerated decline in renal function [8]. Studies on uraemic patients have shown a multitude of atherogenic risk factors such as hypertension and abnormal lipid metabolism, in addition to diabetes mellitus and hyperparathyroidism [32]. Higher plasma calcium in ADPKD patients than in the control group (Table 4), might be due to cystic calcification and renal osteodystrophy [32]. Cystic calcification might be the consequence of secondary hyper parathyroidism, a recognized complication in patients with renal failure as reported by Coffin et al. (1999) [33].
In chronic kidney disease (CKD), waste products build to high levels when untreated and result in complications such as high blood pressure, anemia and weak bones [23]. The observed increase in the level of serum calcium (Ca) and potassium (K) and decrease in the level of serum sodium (Na) and iron (Fe), might also increase the risk of developing heart and blood vessel diseases that might eventually lead to kidney failure [23]. Iron is needed for healthy blood cells and for overall good health [31]. The kidneys signal the body to make enough red blood cells, and iron helps in making them healthy (National Kidney Foundation).The study has also reported the prevalence of associated complications like diabetic mellitus, hypertension, renal osteodystrophy and anaemia.
From the observations made in the present study it may be concluded that:
1) Abnormalities in basic lipid profile tend to be more frequent in ADPKD and it might constitute a major atherogenic risk factor for the development of diabetes mellitus and cardiovascular disease which might significantly decrease the life span.
2) The changes noted in the levels of calcium, sodium, iron and potassium might be responsible for complications like hyponatremia, hypertension, hypoparathyroidism and renal osteodystrophy in ADPKD patients among south Indian (Madurai) population.
The limitation of the study is that the samples were collected only from the patient who came from areas in and around madurai district to take treatment at the Kidney Transplantation & Research Centre and Government Rajaji Hospital in Madurai.
The future study will be focused on PKD1 and PKD2 gene polymorphism.
NOTES