Nitric Oxide: The Key Molecule for Polyphenols Antimicrobial Action ()
1. Introduction
Reactive Oxygen Species (ROS), and Reactive Nitrogen Species (RNS) have been recognized to play a fundamental role in the pathogenesis of several chronic and degenerative diseases, leading to increase the interest in antioxidants as prophylactic or therapeutic agents. To investigate the harmful action of these radical species, most of the work was focusing on vitamin E, ascorbate, and carotenoids [1], but, during the last decade, food polyphenols have received more attention [2] - [6]. Thus, several studies for the identification of polyphenols, better absorbed and/or forming active metabolites have been carried out [7].
To account for polyphenols’ action, NO is often invoked as the active intermediate and, for its production, besides an enzymatic path, an electron transfer process possible even at pH slightly lower than physiological is hypothesized [8] [9] [10] [11]. In particular, previous studies on sulfur derivatives commonly used as antimicrobials, antioxidants and food preservatives [12] [13], showed the ability of these species to act as cofactors of nitrous acid inducing NO release via an E.T. mechanism [14] (Scheme 1(a)). As well, studies conducted on the reaction between p-coumaric, ferulic, and caffeic acid, and acid nitrite (HNO2), reported the formation of nitrous and nitro derivatives through the intermediacy of NO [15] [16] [17] [18]. All these results support the E.T. mechanism and, as well, emphasize the prominent role of acid pHs in the aqueous/biological medium, as demonstrated by the abundant production of NO in the stomach (pH < 2) [19] ; but, the NO production, in very acidic conditions, is believed to proceed through a different mechanism (Scheme 1(b)). In fact, at first, the nitrosonium ion (NO+) is formed, which is rapidly reduced to NO to pass into the gaseous phase (Henry’s law), and then eliminated by expiration. Such a mechanism makes the production and removal of NO very dynamic, and then too low the residual amount of NO in the gastric juice to account for the many biochemical effects attributable to its permeability through the stomach wall. So, other mechanisms of NO production and different mechanisms of action must be involved (Scheme 1).
To understand the role/action of polyphenols, it also needs to consider their chemical structures. In fact, these species are ingested mainly as glycosides, very soluble in water and then quickly excreted, and to obtain free polyphenols, which are the reactive species, it is necessary to a chemical process led by digestive activities and liver changes [20] [21] [22] [23]. It follows that the amount of glycosides that will remain available is low, and therefore the amount of free polyphenols in circulation insufficient to contribute to the total antioxidant capacity. In light of this, it is necessary to stress that the entire antioxidant system, in vivo,
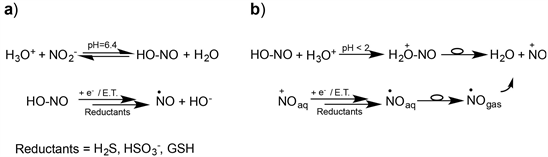
Scheme 1. The mechanism of NO production as a function of pH: (a) At slightly acidic pHs; (b) At very acidic pHs.
is usually in equilibrium, then exogenous antioxidants could play a role only if this equilibrium breaks down [24] [25], and if they are in the right place at the right time. Finally, the ability of exogenous antioxidants to trap ROS largely depends on phenolic hydroxyl groups and, in a biological medium, a sequential proton-loss/electron-transfer appears to be the main route of action [26] ; this lets to consider the transfer of signal, and the protection from microorganisms their primary biological role [25] [27] [28] [29] [30]. In this work, we set out experiments at different substrate concentrations and constant pH, hypothesizing the NO production through an E.T. mechanism [14]. In particular, we studied the effect of polyphenolic compounds, i.e., cinnamoyl derivatives such as the ferulic, caffeic, p-coumaric and sinapic acids, on the concentration of released NO when reacting with an aqueous nitrite solution; to do this, the detection of the adduct of NO with Fe++(DETC)2, by EPR spectroscopy, was used as outlined in the next section. In particular, such a technique allows to detect the formation of NO independently of the medium’s pH, and then even in conditions different from those of compartments in which the NO production seems appointed; this would support the involvement of new pathways for NO formation, and new mechanisms of action (Scheme 2).
2. Material and Methods
2.1. Materials
All experiments have been conducted at room temperature with commercial products, ferulic, caffeic, p-coumaric and sinapic acids, at the highest degree of purity, except the Fe++(DETC)2, iron(II) N, N-diethyldithiocarbamate, which was synthesized as follows: 25 mL of a water solution of diethyldithiocarbamate (DETC), 20 mmol/L, were added to 100 mL of a water solution of FeSO4∙7H2O, 10 mmol/L, over a 60-min period. The mixture was stirred for two hours, and the precipitate, Fe++(DETC)2, collected by filtration; the process was conducted in a rigorous nitrogen atmosphere. The buffer solution was freshly prepared, and the solvents, CH2Cl2 and CH3OH, spectroscopic degree.
2.2. EPR Experiments
Samples were prepared using a device formed by two vials, equipped with a
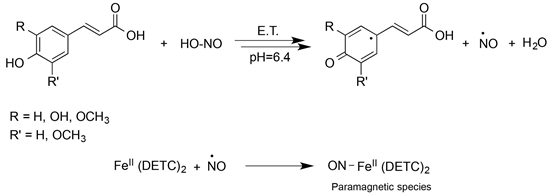
Scheme 2. Reaction between hydroxycinnamic acids and HNO2, in light acidic conditions.
porous septum and connected by means of a tiny PTFE tube; in the first vial, 3.0 mL of a buffer solution (pH = 6.4) of NaNO2 (1.0 mM) and variable amounts of polyphenols, in ratio 1:3:5, were reacted. The second vial was filled with 3.0 mL of a CH2Cl2 solution of the spin trap, Fe++(DETC)2, (3.0 mM). In particular, methanolic stock solutions of ferulic, caffeic, and p-coumaric acids were prepared and, from these, reference solutions at concentration of 1.29 mM, 3.86 mM, and 6.44 mM, i.e., in ratio 1:3:5. For the sinapic acid, because of its poor solubility in water, the molar extinction coefficient was at first determined by UV-spectroscopy, ε324 = 26849 mol−1·dm3·cm−1, and solutions at 2.4 × 10−3 mM, 7.0 × 10−3 mM, and 11.70 × 10−3 mM, i.e., in ratio 1:3:5, were then prepared. Concerning chlorogenic and caffeic acid, experiments with solutions 5.0 × 10−3 mM concentration, under the same experimental conditions used for all the substrates, have been carried out; finally, to check if the production of NO can be deriving even from other sources, a blank experiment, i.e., in the absence of polyphenols, was carried out. All samples were prepared by bubbling a stream of N2-gas into the first vial to allow polyphenols to react with NaNO2; afterward, the N2 stream, rich in NO picked up from the first vial, is forwarded into the second vial containing the methylenic trap-solution, in which the paramagnetic adduct, NO-Fe++(DETC)2, can be formed. In particular, NO was collected, passing through this solution the N2/NO stream for 20 minutes, and then used to carry out the EPR experiments. So, a small aliquot of the solution was introduced into a capillary EPR test-tube and investigated through an EM Brüker EPR spectrometer; the spectra, analyzed through the Win-EPR software (Bruker), were then compared with each other using Microsoft Excel software. Finally, it needs to stress that all experiments were conducted under an inert atmosphere, N2 gas, for avoiding the possible oxidation of NO.
3. Results and Discussion
3.1. Establishing the Production of NO
As hypothesized in the introduction, for supporting the release/formation of NO through an E.T. mechanism, EPR experiments, using spin-trapping techniques, were conducted.
1) An acidic buffer solution (pH = 6.4) of nitrite was reacted with polyphenols such as ferulic, caffeic, p-coumaric and sinapic acids; all experiments allowed to detect an intense EPR signal ascribable to the paramagnetic adduct of NO to the spin trap, NO-Fe++(DETC)2. This supported both a non-enzymatic mechanism and the different capacity of each substrate in inducing the release of NO [31] (Figure 1).
2) Relationship between Hydroxycinnamic acids concentration and NO release.
Since the effectiveness of polyphenols as NO inducer might depend on the available amount of free hydroxycinnamic acid and not of glycosides, esters or amides, i.e., the ingested parent species, EPR experiments with pure hydroxycinnamic acids, at variable concentration, have been carried out. In particular, a
![]()
Figure 1. EPR spectra (stacked area) of NO-Fe++(DETC)2, aN = 1.28 mT and g = 2.039, detected when reacting the hydroxycinnamic acids (same concentration, 6.44 mM) with the reference acidic nitrite solution. Blank Experiment: no polyphenol was present.
buffer solution (pH = 6.4) of nitrite was reacted with methanolic solutions of ferulic, p-coumaric, caffeic and sinapic acids, at three different concentrations in ratio 1:3:5 (Figure 2).
The correlation between the amount of NO released/trapped, EPR spectrum area, and the concentration of pure hydroxycinnamic acid used, undoubtedly prove the different intrinsic capacity of each polyphenol to act as NO inducer (Figure 3).
3) The role of the medium.
The behavior/function of polyphenols often refer to experiments in vitro, and in solvents not com-parable to a biological medium, therefore, different will be their solubility, and then the available amount of free hydroxycinnamic acids. To verify this, we focused our attention on sinapic acid, which is considered both a powerful antioxidant and an antimicrobial agent [31] [32]. Two stock solutions, one aqueous and one methanolic, in which the sinapic acid solubility is markedly different, were prepared. EPR experiments were carried out by reacting these solutions, at three different concentrations, in ratio 1:3:5, with a buffer solution (pH = 6.4) of nitrite; all experiments led to the detection of the paramagnetic adduct of NO to the trap, NO-Fe++(DETC)2 (Figure 4).
Comparing EPR spectra, a direct correlation between the amount of NO released/detected and that of free hydroxycinnamic acid available, independent of the solvent, can be confirmed [32] ; but, the role of the solvent is underlined too, in fact, it manages the quantity of available substrate through the solubility (Figure 5).
![]()
Figure 2. EPR spectra (stacked areas) of the radical NO-Fe++(DETC)2, detected with four different hydroxycinnamic acids; three different methanolic solutions at concentrations, in ratio 1:3:5 = (L) 1.29 mM:(M) 3.86 mM:(H) 6.44 mM, were tested. We used methanolic solutions because in this solvent all substrates are soluble enough.
![]()
Figure 3. Relative amounts of NO-released as a function of the available free hydroxycinnamic acid. Values are standardized to the lowest detected amount of NO, among all experiments, i.e., [caffeic acid] = 1.29 mM.
3.2. NO, Probably Mediator of the Antioxidant Action as Well?
Since the formation of NO is the first step of reaction between hydroxycinnamic acids and acidic nitrite [33] [34] (Scheme 2), beside the antimicrobial action might be plausible to hypothesize NO as the key-molecule even for the antioxidant
![]()
Figure 4. EPR spectra, (stacked area), of NO-Fe++(DETC)2. Sinapic acid from different stock solutions, CH3OH (M) and H2O (W), at different ratio of concentration: 1:3:5 = [L] Low:[M] Medium:[H] High.
![]()
Figure 5. NO released from samples of sinapic acid at different concentrations, in different solvents. (¿) CH3OH [mM] and (¢) H2O [mM] × 10−3. All concentrations used are in the limit of solubility of sinapic acid in water and methanol.
role, i.e., responsible for this action. To support or disclaim this hypothesis, substrates as the chlorogenic and the caffeic acid, widely recognized to be excellent antioxidants, were investigated [35] [36]. EPR experiments, conducted under the same experimental conditions, showed the formation of the paramagnetic adduct, NO-Fe++(DETC)2, with both substrates, but the NO detected/released by the chlorogenic acid was prevailing over that of caffeic acid, (5.9:1.0), in agreement with its greater antioxidant capacity (Figure 6).
However, since chlorogenic acid also has antimicrobial action, and NO is the main detectable product of the process, it seems reasonable to consider NO involved/responsible for both, the antimicrobial and the antiradical action [37]. This behavior seems to be common to all the investigated hydroxycinnamic acids, thus allowing to consider NO as the active species on behalf of hydroxycinnamic acids actions.
3.3. The Hypothesized Mechanism of Action
Several methods, for evaluating the antioxidant capacity of polyphenols, and therefore their anti-radical activity [38], are reported in the literature; among these, the Trolox equivalent antioxidant capacity (TEAC), the DPPH radical scavenging activity, the Efficient Quantity (EC50) [31] [39] - [43], and so on, but all parameters obtained with these techniques result quite scattered if compared each other, i.e., non-coherent. Furthermore, it needs to stress that such values are obtained carrying out experiments in different organic solvents in mixture with water, which strongly influences the final result [38] [39]. More reliable and comparable values for measuring the antimicrobial capacity could be the redox potential [39] [40], even if for these parameters the values are depending on solvent, acidity, etc. Concerning our experiments, other undisclosed processes can be involved and contribute to the final results, such as the dimerization or higher
![]()
Figure 6. Quantitative comparison of NO released reacting chlorogenic acid (¢) and caffeic acid (¢), same concentration, with a reference solution of acidic nitrite (HNO2).
polymerization of phenoxy intermediates, which could lead to products still acting as antioxidants and/or antimicrobials [32] [41] [44] as reported for ferulic and coumaric acids present in food [33]. In fact, by thermal processing such as sterilization or cooking, the formation of dimers comes evident, and their antioxidant and antimicrobial action are higher than that of the parent compound [45] [46] [47].
4. Conclusion
By EPR spectroscopy we have demonstrated that natural polyphenols, as hydroxycinnamic acids, recognized to have both antimicrobial and antiradical action, can carry out these actions through the NO intermediacy. In fact, the interaction of polyphenols with a buffer solution (pH = 6.4) of acidic nitrite (HNO2), suggests an efficient NO release, and this accountable only via non-enzymatic process. Such a mechanism, for the NO production in vivo, was already reported, but, in a very acidic medium as into the stomach and the nitrosonium ion the reactive intermediate. This appears unlike since the antimicrobial action of polyphenols can take place even in compartments characterized by very different pH values, as supported by studies conducted in vivo on different types of bacteria, and the presence of NO seems necessary, but this achievable only via a non-enzymatic (Electron Transfer) process. Some experiments showed a different efficiency in inducing NO release, i.e., not accountable through the polyphenol’s redox potential, and the solubility, as mainly considered; considering this, as suggested in the literature, we assumed that by-products deriving from the polymerization of polyphenols’ intermediates are involved, and reducing agents more potent than the parent polyphenols. This study also led to reconsider the antiradical action of polyphenols, as this action might depend on the intermediacy of NO, the main reaction product, and this proved by the study conducted on antioxidants as the chlorogenic and caffeic acids, for testing their NO inducers capability. In definitive, both the antimicrobial and the antioxidant activity of hydroxycinnamic acids can take place even in mildly acidic conditions, and NO the active molecule, whose formation is conceivable only through a non-enzymatic mechanism.
Acknowledgements
We thank Prof. A. Arcioni, Bologna University, for the Bruker EM-EPR Spectrometer facility.