1. Introduction
Extracellular polysaccharides are attracting increasing attention in the field of medicine because of their positive biological activity and potential application value [1]. Research revealed that extracellular polysaccharides produced by two marine microorganisms, Fusarium oxysporum and Aspergillus ochraceus, showed strong anti-lipid peroxidation ability and free-radical (e.g. superoxide anions) scavenging potential [2] and extracellular oligosaccharides of an endophytic bacteria (14-DS-1) found in Codonopsis pilosula activate macrophages of the immune system and affect their migration ability and microfilament morphology [3]. Moreover, evidence indicates that they can affect normal spindle formation and induce cells to tend to stagnate in the S phase and increase the apoptotic rate. One possible mechanism to achieve this effect would involve the capacity of extracellular polysaccharides to affect spindle-related precise regulation and DNA synthesis during the cell cycle [3]. Similarly, antineoplastic polysaccharides extracted from mushrooms were shown to enhance the immune response in vivo and in vitro and act as a modifier of biological reactions [1].
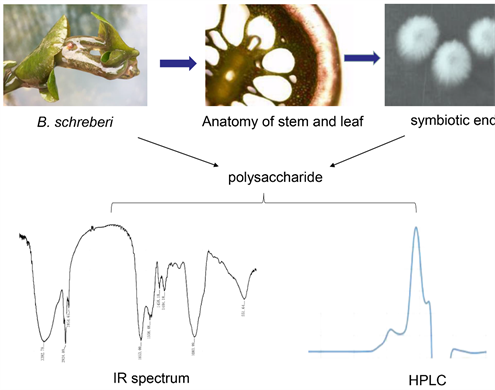
Recent studies have revealed the promising clinical role of extracellular polysaccharides from multiple plants [4] [5], including Brasenia schreberi [6] [7]. B. schreberi, also known as water shield, horseshoe, lake vegetable, water anemone, sunflower and lotus leaf or cauliflower, is a perennial floating-leaf aquatic plant belonging to the family Nymphaeaceae [8] [9]. The main component of the viscous mucilage on the surface of B. schreberi is a viscous polysaccharide [10] with exceptionally high nutritional value and protection role [11]. As an indicator plant of the environment in wetlands, B. schreberi growth demands a high-quality water [12]. However, in recent years, habitat fragmentation and loss and human disturbance have led to a higher organic content in the original and wild water environment, causing a deterioration in the wild-living condition of B. schreberi [12]. Moreover, B. schreberi has been recently listed as a critically endangered species in several countries of East Asia [13]. In this context, seeking an alternative method to produce B. schreberi mucilage polysaccharide is of top priority.
Given that plants and their symbiotic endophytes can produce the same or similar functionally active ingredients, endophytes may be an important source of new active natural products [14]. Endophytes are microorganisms that survive in healthy plant tissues during part or all of their life cycles without causing obvious infection symptoms in their host. For example, previous results showed that Erwinia sp. SS2, an endosymbiotic bacterium found in Chinese yam, showed the ability to produce extracellular polysaccharides [15]. Further, a previous study showed that there are many gum-producing bacteria in the viscose polysaccharide of B. schreberi [16]. To comprehensively utilize B. schreberi and seek alternative bacteria that can produce mucilage polysaccharide similar to B. schreberi, this study aimed to isolate potential symbiotic bacteria from B. schreberi and study their polysaccharide-producing characteristics.
2. Materials and Methods
2.1. Experimental Materials
Stems and leaves of B. schreberi were obtained from Fubaoshan Nature Reserve in Lichuan, Hubei Province. These were rinsed with 30% hydrogen peroxide and then with distilled water for further use.
2.2. Isolation of Endophytic Bacteria from B. schreberi
Stems, leaves and roots of B. Schreberi were sterilized with 0.1% mercury for 10 min and rinsed with distilled water several times in a sterile environment. Thereafter, these were inoculated on LB solid medium and cultured in the dark. Three kinds of well-growing colonies were selected from the medium and transferred to the new LB medium plate. The colonies were purified and numbered as No. 1, No. 2 and No. 3. In addition, the flourishing bacteria were preserved in glycerol suspension for subsequent experiments.
2.3. Identification of Endophytic Bacteria in B. schreberi
The 16S rDNA sequence of bacteria (27F/1492R primer) or ITS sequence of fungi (ITS1 and ITS4 primer) were amplified based on standard procedure of bacteria/fungi identification, which is exerted by. The amplified products were sequenced by the Sanger method. The sequencing results were compared and analyzed on NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi). Cases of homology greater than 97% and highest homology were used to identify the candidate species of endophytic bacteria.
16S rDNA sequence amplification primer sequence:
Primer 1 27F: 5-AGAGTTTGATCCTGGCTCAG-3'
Primer 2 1492R: 5'-TACGGCTACCTTGTTACGACTT-3'
The size of amplified fragments is approximately 1400 - 1700 bp
ITS sequence amplification primer sequence:
Primer 1 ITS1: 5'-TCCGTAGGTGAACCTGCGG-3'
Primer 2 ITS4: 5'-TCCTCCGCTTATTGATATGC-3'
The size of amplified fragments is approximately 400 - 700 bp
2.4. Purification and Extraction of Polysaccharides from the Endosymbiont
Strains were selected from the plate of LB medium and transferred to the purified strains. The strains were inoculated into the liquid LB medium. The fermentation liquid was centrifuged for 5 min at 200 rpm and incubated at 37˚C overnight for 2 days. The supernatant was obtained after centrifugation at 12,000 rpm for 5 min. Next, the supernatant and the organic solution were mixed with chloroform-n-butanol solution (chloroform: n-butanol = 4:1) at a 3:1 ratio for extraction in a centrifugal tube. The supernatant was oscillated for 20 - 30 min and centrifuged at 8000 rpm for 10 min. Precipitated protein between the two phases could not be discarded; thus, it continued to be removed five times according to the above operation. Then, the supernatant was filtered twice with a 0.45-μm micron membrane. After filtration, it was heated and concentrated to 1/2 - 2/3 of its original volume at 70˚C. Thereafter, it was added with a 4-fold volume of 95% ethanol. Finally, the supernatant was frozen overnight in a refrigerator and centrifuged at 12,000 rpm for 10 min. The precipitate was considered the endophytic bacterial polysaccharide.
2.5. Extraction and Separation of Crude Polysaccharide from B. schreberi
Tender sprouts of B. schreberi were extracted in NaOH (0.1 mol/L); the ratio of material to liquid was 1:2 (w/v) and the pH was adjusted to 7.0 after 1.5 h of alkali extraction at 25˚C. The sprouts were removed, the residues were separated by centrifugation and the supernatant was concentrated at 60˚C for 1 h using a magnetic stirrer. Thereafter, the Sevage method was used to treat the concentrated crude extract repeatedly to remove the free protein and concentrate again to approximately 2/3 of the original volume. A 4-fold volume of absolute ethanol was added to precipitate overnight; thereafter, the mixture was centrifuged for 10 min at 4000 rpm to obtain the precipitate. Next, a small amount of water was used to dissolve the precipitate, which was then concentrated and added with a 4-fold volume of ethanol to precipitate overnight. This operation was repeated three times before final drying to obtain the B. schreberi polysaccharide (BSP).
2.6. Determination of Sugar Content in the B. schreberi Polysaccharide Extract
Polysaccharide content of BSP was determined by the phenol-sulfuric acid method and D-glucose as standard. First, D-glucose was used to prepare the standard curve solution (0.1 mg/ml). Second, the reference solutions were placed into the test tubes (0.2, 0.4, 0.6, 0.8 and 1.0 ml) and the phenol solution was added to stabilize them. Then, concentrated sulfuric acid was quickly added for shaking. The tubes were allowed to stand for 10 min until cooled to room temperature and then the absorbance was measured at 490 nm. Double distilled water (DDW) was used as the blank control. The results were analyzed by linear regression analysis. Thereafter, the absorbance level of the sample was measured to determine the content of glucuronic acid.
2.7. Structural Analysis of Polysaccharides from B. schreberi and Comparison with Polysaccharides from Endophytes
The chemical composition of polysaccharide samples was determined by high performance liquid chromatography (HPLC) using an Agilent 1260 Infinity II LC System equipped with a Shodex OH-pak SB-804 HQ column (8 mm × 300 mm). To further compare the glycosidic bonds of the polysaccharides between symbiotic bacteria and B. schreberi, the structure of polysaccharides was further analyzed by Fourier transform infrared spectroscopy (IR) (IRTracer-100).
2.8. Determination of Antioxidant Activity of Polysaccharides
In this experiment, the hydroxyl radical kit provided by Nanjing Jiancheng Bioengineering Institute was used to determine polysaccharide activity. The polysaccharide sample and reagent were mixed evenly and reacted at 37˚C for 1 min, after which the color reagent was added immediately to stop the reaction. After mixing evenly, the polysaccharide sample was placed at room temperature for 20 min. The spectrophotometer was zeroed with distilled water and the absorbance of each tube was measured at 550 nm. The experimental results showed the scavenging of hydroxyl radicals and some antioxidant activities of the polysaccharides obtained.
3. Results
3.1. There are Well-Developed Airways and Glandular Cells in the Stems and Leaves of B. schreberi
The stomata were distributed on the upper epidermis of the leaves (Figure 1(A)). The lower epidermis of the leaves was in contact with water, so there were more stomata on the upper epidermis for photosynthesis and gas exchange. Mucilage was abundant on the dorsal surface of the stem and the leaf and there were many black stripes on the lower epidermis of the leaf. Study showed these structures were glandular cells. In addition, red dots and black stripes were observed, which might be symbiotic bacteria (Figure 1(B)). There was a layer of pectin on the surface of the stem of fresh water shield. Under this pectin layer, there were glandular cells with a hollow airway in the middle to allow gas exchange (Figure 1(C)). After incubation in the laboratory for a period of time, the glandular cells disappeared and there was no pectin on the surface of the stem (Figure 1(D)). Therefore, pectin secretion may be related to glandular cells.
3.2. Isolation of Endophytes
The three colonies grew well in the LB medium plate. Among them, the diameter of colony No. 1 was approximately 1 - 2 mm, its shape was regular, round, smooth and tidy edged and was wet and milky yellow, with a transparent surface (Figure 2(A)); the diameter of colony No. 2 was approximately 4 - 6 mm, the colony bulged, the edge was not smooth, the hypha was visible and the color was opaque white (Figure 2(B)); the diameter of colony No. 3 was 5 mm and the colony was round and milky white (Figure 2(C)).
3.3. Symbiosis of Bacteria and Fungi with B. schreberi
According to the standard sequencing method of bacteria and fungi, the sequence of symbiotic bacteria B. schreberi was as follows:
![]()
Figure 1. Transverse section of the stem and the leaf of Brasenia schreberi. (A) An upper epidermis of the leaf; (B) Lower epidermis of the leaf; (C) Transverse section of a fresh stem; (D) Transverse section of a stem grown in culture.
![]()
Figure 2. Morphology of the colonies of the three endophytic bacteria from Brasenia schreberi. (A) colony No. 1; (B) colony No. 2; (C) colony No. 3 (Bars = 2 mm).
No. 1:
GCATGGCAGCGCTAGAGTTGCTCCTGGAGCGGAAGGCCCTTCGGGGTACTCGAGCGGCGAACGGGTGAGTAACACGTGAGTAACCTGCCCCAGGCTTTGGGATAACCCTCGGAAACGGGGGCTAATACCGAATATGACCTCCGGACGCATGTTTGGTGGTGGAAAGTTTTTCGGCCTGGGATGGGCTCGCGGCCTATCAGCTTGTTGGTGGGGTGATGGCCTACCAAGGCGACGACGGGTAGCCGGCCTGAGAGGGCGACCGGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGGGCGGAAGCCTGATGCAGCGACGCCGCGTGAGGGATGACGGCCTTCGGGTTGTAAACCTCTTTCAGCAGGGACGAAGCGTAAGTGACGGTACCTGCAGAAGAAGCGCCGGCCAACTACGTGCCAGCAGCCGCGGTAAGACGTAGGGCGCGAGCGTTGTCCGGATTTATTGGGCGTAAAGAGCTCGTAGGCGGCTTGTCGCGTCGACCGTGAAAACTTGGGGCTCAACCCCAAGCCTGCGGTCGATACGGGCAGGCTAGAGTTCGGTAGGGGAGACTGGAATTCCTGGTGTAGCGGTGAAATGCGCAGATATCAGGAGGAACACCGGTGGCGAAGGCGGGTCTCTGGGCCGATACTGACGCTGAGGAGCGAAAGCGTGGGGAGCGAACAGGATTAGATACCCTGGTAGTCCACGCTGTAAACGTTGGGCGCTAGGTGTGGGGGGCCTCTCCGGTTCCCTGTGCCGCAGCTAACGCATTAAGCGCCCCGCCTGGGGAGTACGGCCGCAAGGCTAAAACTCAAAGGAATTGACGGGGGCCCGCACAAGCGGCGGAGCATGCGGATTAATTCGATGCAACGCGAAGAACCTTACCTGGGTTTGACATGGCCGCAAAACCTCCAGAGATGGGGGGTCCTTCGGGGGCGGTCACAGGTGGTGCATGGCTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTCGTTCGATGTTGCCAGCGCGTTATGGCGGGGACTCATCGAAGACTGCCGGGGTCAACTCGGAGGAAGGTGGGGATGACGTCAAGTCATCATGCCCCTTATGTCCAGGGCTTCACGCATGCTACAATGGCCGGTACAATGGGCTGCGATACCGTGAGGTGGAGCGAATCCCAAAAAGCCGGTCTCAGTTCGGATCGGGGTCTGCAACTCGACCCCGTGAAGTCGGAGTCGCTAGTAATCGCAGATCAGCAACGCTGCGGTGAATACGTTCCCGGGCCTTGTACACACCGCCCGTCACGTCACGAAAGTCGTCAACGTAGGAAGTGCGGTGGCCCCACCCTGGTGGAGGCAACGTTAGAGTAGAGCTATCCGCCC
No. 2:
GTAGGTTGAACCTGCGGAGGGATCATTACTGAGTTCTACAAAAAACTCCCAACCCTTTGTGAACCTTACCGTCGTTGCCTCGGCGCCGAGCGGCGGCTACCCTGGAGAAGCTACCCGGGAGCCACCTACCCTGTAGGTGGCTACCCTGGAGCTACCCTGTAGTAGTTTGCATTCTACGCTCCGCCGGCGGACCTTCTACACTCTGTTTTGTATAGTGTATCTCTGAAACCTATAACGTAATACGTTAAAACTTTCAACAACGGATCTCTTGGTTCTGGCATCGATGAAGAACGCAGCGAAATGCGATACGTAATGTGAATTGCAGAATTCAGTGAATCATCGAATCTTTGAACGCATATTGCGCCCATTAGTATTCTAGTGGGCATGCCTATTCGAGCGTCATTTCAACCCTTACGCCCCTGTTGCGTAGTGTTGGGAACCTACAGGCCTGTAAAAAGGACCTGTAGCTCCCTAAAGGTAGTGGCGGTGTTAGGTACACTCGTAGCGTAGTAACATCTTTTCTCGCTCCTGCAGTGTACCTAAGGCCTGCCGTGAAAAACCCCCTATAACTTCTAGTGGTTGACTTCGGATTAGGTAGG
No. 3:
GAATGGCGGGCCGCTATAATGCTAGTCGAGCGAACAGATAAGGAGCTTGCTCCTTTGACGTTAGCGGCGGACGGGTGAGTAACACGTGGATAACCTACCTATAAGACTGGGATAACTTCGGGAAACCGGAGCTAATACCGGATAACATATTGAACCGCATGGTTCAATAGTGAAAGGCGGCTTTGCTGTCACTTATAGATGGATCCGCGCCGTATTAGCTAGTTGGTAAGGTAACGGCTTACCAAGGCAACGATACGTAGCCGACCTGAGAGGGTGATCGGCCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTAGGGAATCTTCCGCAATGGGCGAAAGCCTGACGGAGCAACGCCGCGTGAGTGATGAAGGTCTTCGGATCGTAAAACTCTGTTATCAGGGAAGAACAAATGTGTAAGTAACTGTGCACATCTTGACGGTACCTGATCAGAAAGCCACGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGTGGCAAGCGTTATCCGGAATTATTGGGCGTAAAGCGCGCGTAGGCGGTTTTTTAAGTCTGATGTGAAAGCCCACGGCTCAACCGTGGAGGGTCATTGGAAACTGGAAAACTTGAGTGCAGAAGAGGAAAGTGGAATTCCATGTGTAGCGGTGAAATGCGCAGAGATATGGAGGAACACCAGTGGCGAAGGCGACTTTCTGGTCTGTAACTGACGCTGATGTGCGAAAGCGTGGGGATCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAGTGCTAAGTGTTAGGGGGTTTCCGCCCCTTAGTGCTGCAGCTAACGCATTAAGCACTCCGCCTGGGGAGTACGACCGCAAGGTTGAAACTCAAAGGAATTGACGGGGACCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAAGAACCTTACCAAATCTTGACATCCTTTGACCGCTCTAGAGATAGAGTCTTCCCCTTCGGGGGACAAAGTGACAGGTGGTGCATGGTTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAGTCCCGCAACGAGCGCAACCCTTAAGCTTAGTTGCCATCATTAAGTTGGGCACTCTAAGTTGACTGCCGGTGACAAACCGGAGGAAGGTGGGGATGACGTCAAATCATCATGCCCCTTATGATTTGGGCTACACACGTGCTACAATGGACAATACAAAGGGCAGCTAAACCGCGAGGTCAAGCAAATCCCATAAAGTTGTTCTCAGTTCGGATTGTAGTCTGCAACTCGACTACATGAAGCTGGAATCGCTAGTAATCGTAGATCAGCATGCTACGGTGAATACGTTCCCGGGTCTTGTACACACCGCCCGTCACACCACGAGAGTTTGTAACACCCGAAGCCGGTGGAGTAACCATTTATGGAGCTAGCCGTCGAGTGGAGAGCTCAGTCT
The results of sequencing were compared and analyzed via browsing the NCBI website. The species with over 97% homology and highest homology were finally identified as endosymbiotic species. The results showed that two of the symbiotic bacteria isolated were bacteria (colonies No. 1 and No. 3), while the other was a fungus (colony No. 2) (Table 1). Endophytes were identified as Micromonospora sp.YG-1, Xylariaceae sp. strain UT-X and Psychrobacter pulmonis strain T-15.
3.4. Extracts from the Fermentation Broth of Symbiotic Bacteria Found in B. schreberi Contained Secreted Polysaccharides
Polysaccharide content in the fermentation broth of endophytic bacteria from B. schreberi was determined and compared. After polysaccharide was hydrolyzed by sulfuric acid, the content of secreted sugar in the bacterial solution was determined by DNS (3-amino-5-nitro salicylic acid) method. With D-glucuronic acid as the reference standard, the standard curve was drawn by the absorbance of different concentrations of standard glucose solution reacted with DNS reagent and sulfuric acid. The standard curve was y = 0.0368x − 0.0436 (R2 = 0.9807). The content of polysaccharides was determined according to this standard curve (Table 2). The results showed that the polysaccharide contents in the fermented extracts were as follows: 0.30 ± 0.01 mg/ml in B. schreberi samples, 0.07 ± 0.001 mg/ml in Micromonospora sp.YG-1, 0.07 ± 0.002 mg/ml in Xylariaceae sp. strain UT-X and 0.06 ± 0.001 mg/ml in Psychrobacter pulmonis strain T-15 (Table 2).
![]()
Table 1. Species identification of three endophytic bacteria.
![]()
Table 2. Determination of polysaccharides from different species.
3.5. Organic extracts of Endophytic Bacteria from B. schreberi Have Similar Peaks as Those of Polysaccharides from B. schreberi by HPLC
We used HPLC to compare polysaccharide components detected in the bacterial species identified. HPLC spectra showed that the polysaccharide extracts from Micromonospora sp.YG-1 had a peak at 19 min and a sub-peak at 21 min (Figure 3(A)). Polysaccharide extracts from the other two endophytic bacteria, Xylariaceae sp. strain UT-X and Psychrobacter pulmonis strain T-15, also showed similar peaks (Figure 3(B), Figure 3(C)). Interestingly, the sub-peak at the 21st min was similar to the peak signal of the polysaccharides extracted from B. schreberi (Figure 3(D)), which suggests that symbiotic bacteria in B. schreberi can produce polysaccharides similar to B. schreberi polysaccharides, besides producing their species-specific polysaccharides.
3.6. Polysaccharides Secreted by Three Endophytic Bacteria in B. schreberi Have Infrared Absorption Peaks Similar to Those of B. schreberi
To further determine whether polysaccharides from endophytic bacteria and those from B. schreberi contain the same chemical functional groups, we performed FTIR (Fourier Transform infrared spectrum) analysis. Infrared spectrum analysis showed that the three symbiotic bacteria had similar infrared absorption peaks. Representative polysaccharides from Psychrobacter pulmonis strain T-15 showed nine characteristic absorption peaks (Figure 4). From the infrared spectra, it can be seen that the peak at 3392.79 cm−1 is the stretching vibration absorption peak of -OH, while that at 2924.09 cm−1 is the stretching vibration absorption peak of C-H; these two are peaks that are characteristic of polysaccharides and similar to those of B. schreberi polysaccharides (Table 3). Peaks at 2854.65 cm−1, 1558.00 cm−1 and 1458.18 cm−1 are the stretching vibration
![]()
Figure 3. HPLC analysis of polysaccharides and secreted metabolites from Brasenia schreberi and its endophytic bacteria. (A) Micromonospora sp. YG-1; (B) Xylariaceae sp. strain UT-X; (C) Psychrobacter pulmonis strain T-15; (D) Brasenia Schreberi.
![]()
Figure 4. Infrared spectrum (IR) analysis of polysaccharides from Psychrobacter pulmonis strain T-15.
absorption peaks of C-H on the benzene ring, which are characteristic spectrum peaks of polysaccharides that are specific of symbiotic bacteria (Table 3); the peak at 1653.00 cm−1 is the carbon-oxygen double bond (C=O) absorption peak; the peaks at 1404.18 cm−1 and 551.64 cm−1 are all C-H stretching vibration
![]()
Table 3. Characteristic peak analysis of polysaccharides from different species.
absorption peaks. Notably, the absorption peak at 1083.99 cm−1 is associated with the C-O-H bond, which is the characteristic peak of beta-glucan (Table 3).
In addition, the polysaccharide produced by the symbiotic bacteria had the same absorption peaks as those of B. schreberi at 3392.79 cm−1 and 2924.09 cm−1 (Table 3), as well as unique characteristic peaks (2854.65 cm−1, 1558.00 cm−1 and 1458.18 cm−1). Further, polysaccharides from symbiotic bacteria and from B. schreberi showed the same characteristic peaks of beta-glucan at 1083.99 cm−1 (Table 3).
3.7. Polysaccharides from Different Species Show Significant Inhibitory Effects on the Production of Hydroxyl Radicals
The importance of polysaccharides lies in their physiological activities. The hydroxyl radical (OH) is a type of free radical with strong oxidation ability. Its chemical nature is very active and its oxidation rate of various organic and inorganic substances is very high. It is the main factor causing lipid peroxidation, nucleic acid and protein breakdown and polysaccharide decomposition in tissues. It is related to aging, cancer, radiation damage and cell phagocytosis. To determine the biological activity of polysaccharides from symbiotic bacteria present in B. schreberi, the antioxidant activity of their polysaccharides was evaluated by analyzing the hydroxyl radical production system. The results showed that the inhibitory effect of polysaccharides from symbiotic bacteria on hydroxyl radical production was concentration-dependent and maximum at 1.0 mg/ml (Table 4). The free radical scavenging effect of polysaccharides from symbiotic bacteria on hydroxyl radicals was significantly higher than that of polysaccharides from B. Schreberi.
![]()
Table 4. Inhibitory effect of polysaccharides from different species on hydroxyl radical production.
4. Discussion
B. schreberi is an economically important aquatic plant that requires high-quality water [12] [13]. It is becoming increasingly pertinent to pursue an alternative way for the production of polysaccharides from water shield due to the continuous deterioration of its natural environment [12]. A distinctive anatomical feature of water shield is the distribution of stomata mainly on the adaxial leaf surface and the abundance of glandular cells on the abaxial leaf surface (Figure 1). In the transverse section of the stem, there is a thick layer of pectin on the surface and many glandular cells [17] It is preliminarily inferred that pectin is the product of glandular cell secretion and that the species’ characteristic polysaccharide may be secreted by glandular cells or by endosymbiotic bacteria. In the present study, three species of bacteria were successfully isolated from sterilized stem and leaf tissues of B. schreberi and the polysaccharides they produce were studied. Three kinds of symbiotic bacteria were isolated and identified, in addition to one fungus (Figure 2, Table 1), reflecting the diversity of symbionts hosted by B. schreberi.
To determine whether the fermentation culture of symbiotic bacteria from B. schreberi contained polysaccharides, the polysaccharide content of the extract medium was determined by the DNS (3-amino-5-nitro salicylic acid) method and analyzed by HPLC (Table 2, Figure 3). The results confirmed that symbiotic bacteria in B. schreberi produce polysaccharides that show the same peaks on HPLC spectrum (Figure 3). IR showed that the polysaccharides from symbiotic bacteria and those from B. schreberi had similar IR characteristic peaks (Figure 4, Table 3). This is similar to the IR spectra of polysaccharides from the seaweeds, Asparagus [18] and Ganoderma lucidum [19]. This finding indicates that plant polysaccharides have similar or identical chemical functional groups. The function of a polysaccharide is reflected by its activity. The detection of hydroxyl free radicals indicated that polysaccharides from symbiotic bacteria present in B. schreberi remarkably inhibited the production of hydroxyl free radicals in a concentration-dependent manner (Table 4). Other biological activities of polysaccharides need to be further investigated using various cell types.
5. Conclusions
To circumvent the increasing deterioration of original living environment faced by B. schreberi in polysaccharide producing, seeking an alternative method to produce B. schreberi mucilage polysaccharide is of top priority. In this investigation, three endophytic bacteria were isolated and purified from B. schreberi, namely Micromonospora sp. YG-1, Xylariaceae sp. strain UT-X and Psychrobacter pulmonis strain T-15. These endosymbiotic bacteria have the ability to produce polysaccharides with the similar retention time in HPLP analysis and FTIR spectrum that effectively scavenge reactive oxygen species. Our study provides a potential basis and feasible scheme for the comprehensive utilization of symbiotic bacteria in B. schreberi to produce effective natural polysaccharides.
Acknowledgements
This work was supported by the Special Fund for Basic Scientific Research of Central Colleges, South-Central University for Nationalities (CZP17051), National Natural Science Foundation of China (31270361) and Fund for Key Laboratory Construction of Hubei Province (Grant No.2018BFC360).
Abbreviations Used
BSP: Brasenia schreberi polysaccharide
FTIR: Fourier Transform infrared spectrum analysis
HPLC: High performance liquid chromatography
Highlights
The main component of viscous mucilage on the surface of B. schreberi is a viscous polysaccharide with exceptionally high nutritional value.
Three species of symbiotic endophytes of B. schreberi can produce the same or similar functional active ingredients of polysaccharide with the counterpart of B. schreberi.
The endosymbiotic bacteria have the promising application to produce polysaccharides and to circumvent the increasing deterioration of original living environment faced by B. schreberi in polysaccharide producing.
NOTES
#The first two authors contributed equally to this work and can be considered co-first authors.