Cost-Minimization and Budget Impact Analysis of Rituximab SC VS Rituximab IV for Non-Hodgkin’s Lymphoma (NHLs) in Greece ()
1. Introduction
The cost of pharmaceutical treatment for a given disease/patient in general comprises of an obvious part, i.e. the cost of the medication per se, or in simple words, the “price on the box”, as well as of a less obvious part, which refers to the costs of administration of the drug and the management of potential side effects. The issue of non-drug costs of pharmaceutical treatments is more pronounced in a number of severe and life-threatening diseases, such as neoplasms or autoimmune diseases, where the administration of medications follows an intravenous pathway. In such cases, the need of healthcare resource use, such as the time spent within the facility, the time of the staff to overview the procedure the required capital costs to build and maintain infusion facilities and other significant parameters (healthcare professional time, waiting times, productivity loss, patient time in the hospital, duration of the treatment) can result to significant costs for the patients, healthcare system and providers [1] [2] . As a result, it is important to take into consideration the drug administration costs to rationalize resource allocation decisions and improve cost-effectiveness [3] .
The advent of new methods of administration for established medications, for example the development and availability of subcutaneous forms of (previously) intravenously administered medications can result in potential benefits for the patients and healthcare professionals [1] [4] and secure a more cost-effective and less intense health resource use pattern for the healthcare system [5] . In a general framework, the available evidence shows that the administration cost of drugs is considerably higher for complex means of administration, such as intravenous (IV) administration, compared to simpler and less time and resource consuming methods, such as the subcutaneous (SC) injection. The average administration cost is almost six times higher for drugs administered intravenously compared to the SC injection based on 2010 values [3] .
Taking the above into account and bearing in mind the significant constraints that the Greek healthcare system is faced nowadays, both in terms of capacity constraints as well as in terms of reduced healthcare budgets due to fiscal constraints [6] , the purpose of the present study is to investigate whether the introduction of a SC form of administration of a widely used drug for a life-threat- ening disease, could result in savings and increases in the capacity of the system to meet the increased demand for services. The paradigm under survey refers to the use of Rituximab for the treatment of patients with Non-Hodgkin's lymphomas (NHLs) in Greece.
Specifically, this study aims to perform a cost-minimization (CMA) and budget-impact analysis (BIA) of introducing and switching to the Rituximab SC injection for the treatment of NHLs in the Greek clinical setting comparing healthcare professionals’ (HCP) time used in the preparation and administration of IV and SC Rituximab for the treatment of patients with NHLs.
2. Methods
Rituximab SC has been demonstrated as non-inferior compared to Rituximab IV in terms of pharmacokinetics and tolerance [7] , thus the analysis was based on the development of a CMA model that estimates the clinical effects and associated costs for patients that undergo treatment for NHLs with administration of either Rituximab SC or Rituximab IV.
In order to account for the costs associated with each treatment option a questionnaire-based survey was implemented with an aim to provide data regarding the local treatment patterns and the associated resource use with each treatment strategy (IV vs. SC) The questionnaire was developed taking into account the active healthcare practitioners time as well as the patient’s time for the following components of the administration of treatment: IV infusion, SC injection, IV or SC preparation and dispensing, time spent on the infusion site, and monitoring time. The questionnaire was completed by the chief physician and the chief nurse of 8 administration points in the Greek NHS. Apart from the actual cost that is associated with the resource use for each administration strategy, calculations focused also on differences in IV and SC processes with regards to annual nursing time saved and annual chair time saved.
Calculations for the CMA, contain both drug and non-drug costs for each treatment option and estimate the total cost per patient over the full course of treatment. The composition of the total drugs and unit administration costs is based on the following equations:
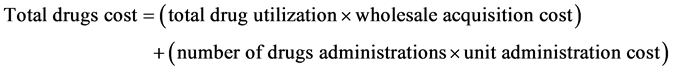

Apart from the CMA, the analysis also considered: a) the BIA of Rituximab SC and Rituximab IV over a 3-year time-period for a hypothetical population of 1000 patients, at different levels of patient shares between the treatment options and b) an impact analysis on the introduction of Rituximab SC in clinical practice in terms of system capacity. The analysis was carried out from the third- party payer perspective, and cost data relevant to personnel salaries, pharmaceuticals and resource utilization were obtained from official government sources and the Greek NHS official unit costs list.
3. Results
According to Table 1, and based on the average values that were produced from
![]()
Table 1. Time differences between the IV and SC administration* as reported by 8 infusion centers in the Greek NHS.
*Average times, as calculated by the mean values of the sample of questionnaires. Values are rounded to the closest integer.
the questionnaires, switching from the IV to the SC administration can lead to time savings for the HCP and the patient (the patient’s stay within the hospital as a result of the procedure). Indicatively, there are significant time differences with regards to the role of the physicians in terms of monitoring during the infusion process (15 minutes for IV compared to 3 minutes for SC administration), whereas important differences were observed in the necessary time between for a series of tasks for the IV and SC administration (Table 1). Most notable changes in the total time of the nurses to complete different processes can be observed in pre-medication administration (3 minutes saved with the use of SC method), monitoring during infusion (10 minutes intravenously compared to 7 minutes subcutaneously). Estimated savings of 5 minutes are also observed in the preparation of the drugs, the actual dispensing process and the post-in- jecting monitoring processes.
The SC administration was associated with a reduction in active HCP time and in time spent in the treatment chair by each patient compared with the IV administration. The results show that the use of SC administration provided a 57% reduction in the nursing time on an average patient (Figure 1) and a decrease of 90% in the chair time (Figure 2). The estimated average chair time per session was 0.32 hours and 3.83 hours for the Rituximab SC and the Rituximab IV, respectively.
The average administration cost per patient over the full course of treatment consisting of eight cycles of therapy and three cycles of maintenance, was 13,627 ?and 14,245 ?for Rituximab SC and Rituximab IV, respectively. Budget savings
for the first year of full implementation of Rituximab SC administration was 618,708?for a hypothetical population of 1000 patients. In addition to clinical and economic benefits, the SC administration could contribute to the increase of the hospital’s capacity to deliver treatments to more patients than before (Figure 3).
4. Discussion
NHLs comprise malignancies which arise from the lymphoid components of the immune system, and account for 90% of all lymphoma cases [8] [9] . Although for most cancers, incidence and mortality rates seem to have decreased during the last decades, a constant rise of NHL cases resulted in the disease being referred to as an epidemic [10] [11] , therefore representing an important burden on health systems consuming economic and societal resources [12] . According to the latest data [13] , 385,741 new cases were reported with NHLs, while 199,670 deaths were recorded in 2012 worldwide.
In order to manage NHLs, several medicinal products have emerged and are currently used by clinical practitioners in Greece and internationally, depending
on the etiology, type and stage of the lymphoma. Rituximab, a treatment option that has demonstrated efficacy for various malignancies, is used in the treatment of NHLs as a standard induction or maintenance therapy for many CD20-posi- tive lymphomas and is usually administered as an IV infusion [14] . This process requires the attention of a number of HCPs to administer the infusion and monitor patients during and after administration, while it leads to long outpatient visits, high resource use and labour-intensive utilization, thus increased corresponding costs [15] [16] . Recently, a SC formulation has been made available, following the demonstration of therapeutic equivalence against the IV form, whilst substantially reducing the administration time that could eventually enhance resource and cost savings [17] .
On the other hand, the administration of IV formulations can also be time consuming for the patients [18] . Switching from IV to SC administration can contribute towards the improvement of patient convenience and experience [5] [18] . The SC administration of Rituximab has led to reduction in HCP time and chair time for each patient compared with the IV administration. According to our results, the SC administration contributed to a significant decrease (57%) in the annual nursing time on the average patient, while a 90% reduction is observed about the annual chair time for the SC compared to the IV route In addition, the SC administration can contribute to the increase of the hospital’s capacity in patients per year as well as in the reduction of costs per patient, a finding that can be attributed mostly to the reductions in resource use, rather than the costs of medication per se, since both medications are priced at parity in Greece.
The results of this study are in general accordance with similar studies of the international literature that aim to estimate the savings that can be generated by alternative forms of drug administration. Indicatively, resource savings due to a potential switch from IV to SC administration of Rituximab have been reported for the UK [18] , Italy [19] , and as well as other European countries [20] , and can be mainly attributed to the reductions in personnel time and the time that the patient spends within the facility according to each of the two methods of administration. This pattern has also been observed with other treatments for other indications in the field of oncology, such as the IV and SC administration for trastuzumab [21] [22] [23] .
5. Conclusion
Acknowledgements
The present study was supported by an unrestricted research grant from Roche Hellas S.A. The authors would like to thank Nadia Boubouchairopoulou and Filippos Tarantilis for their participation and contribution in this study.
Author Contributions
KA, DK and VT contributed to the conception and the design of the study and the acquisition of the data. DK, VT, LP, FT and KA contributed to the analysis and the interpretation of the data. DK and KA drafted the manuscript. DK and KA contributed to the critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Disclosure
The authors declare that they have no conflict of interest.