Comparative Study of Hydrogen Electrooxidation on Gold and Platinum in Solutions Containing Perchlorate Ion ()
1. Introduction
Study of the electrooxidation of hydrogen is of great importance because of their application in oxygen/hydrogen fuel cells. Oxygen electrochemical dissolution is much slower than that of the hydrogen. Therefore, it provides the highest overpotential on the cell operation [1] . It is known that platinum is the metal on which the hydrogen electrode has the highest exchange current density [2] -[5] . However, the kinetic of the hydrogen electrode, because of its speed, suffers interference of material transport and kinetic parameters obtained in acid medium are not reproducible [6] -[8] . Moreover, it is necessary to know the mechanism of the reaction to elucidate the application site of the electrocatalyst in porous media, due to the high cost of the noble metal. On gold, atomic hydrogen diffuses into the metal and its surface concentration is very low [9] -[12] . On both metals potential assisted reconstruction surface occurs [13] . The reappearance of the structure (1 × 1) affects the reaction kinetics. This influence is notably manifested in the case of gold. On the surfaces of both metals, the diffusion of the reagent controls the reaction in conditions of low hydrogen partial pressure [12] [14] .
2. Experimental
A Pyrex glass cell with two compartments is used. In the main compartment the working electrode and the auxiliary electrode are placed. They are constructed with spectroscopic gold or platinum sheets of 5N purity. Apparent area ratio between the auxiliary and working electrode (RHE) is approximately 40/1. A reversible hydrogen electrode, which is used as reference electrode, is placed in the lateral compartment connected to the central compartment by a Luggin capillary. The potentials shown in the figures are referred to the RHE. The electrolyte is a solution of 1M perchloric acid prepared with analytical reagent and degassed with nitrogen of high quality. To study the influence of pH on the reaction, solutions are prepared by mixing perchloric acid and lithium perchlorate, maintaining constant the ionic strength. Rectified hydrogen is used as a reactive. Partial pressure is controlled by mixing the reactive with nitrogen and controlling the flow of each gas by a Matheson flowmeter. The experiments were performed using a LYP potentiostat connected with a ramp function generator of the same brand. The voltammograms were recorded with an Allen XY recorder.
3. Results and Discussion
To study the electrooxidation of hydrogen (H), sweeps between 0.075 and 1.5 V on platinum and between 0.1 to 1.7 V on gold in the absence of the reagent are performed. Then, hydrogen is bubbled at a given partial pressure during the necessary time to obtain a constant H concentration in the electrolyte (30 min). The Scan is then repeated with different potential limits for each metal. The variables studied are the gas partial pressure and the potential sweep rate, V. For both types of surface a linear relationship between the maximum current and the partial pressure approximately fits. The linearity between peak current and square root of the potential sweep rate is found only at low partial pressures, manifesting a diffusion control for those conditions, as shown in Figure 1. For partial pressures greater than 0.1 atm a mixed control probably occurs [12] . On gold, the current drops to zero when the surface reconstruction of (100) plane lifts. On platinum, hydrogen collaborates with the formation of OH groups on the surface, decreasing the current smoothly at potential greater than 0.7 V and dropping sharply at 0.8 V, potential at which the metal oxide appears. The current is anodic in the forward and in the reverse scans for both metals, indicating a strong reaction shift to the right. In the case of Pt, the intermediary is the adsorbed atomic H. For gold, it has been postulated a diatomic cation with a positive charge [12] .
With platinum, sweeping between 0.075 and 1.5 V at 0.1 V/sec, the classic profile of Pt/HClO4 system is obtained. The hydrogen and oxygen monolayer’s formation current can be observed. For studying the oxidation reaction of H2, the sweep between 0.075 and 0.8 V has been done. At first, the scanning in the absence of the reagent is performed. Then, H2 is bubbled at a given partial pressure for a time enough to obtain constant H2 concentration in the solution. Then, the scan to 0.8 V is repeated. It is observed the same voltammogram profile, but moved up as if he had added a constant current. The current difference between the two records was plotted against partial pressure, obtaining a relation that can be approximately fitted to a straight line as shown in Figure 2. The great mass transport contribution on the reaction speed is then evident.
Figure 3 shows voltammetric records obtained with platinum in perchloric acid saturated with hydrogen gas. Blank has been included (no reagent), for comparison purposes. The current is anodic in direct and inverse cycles. Up to 0.75 V, the current is constant and presents typical fluctuations due to convection interference with the media, as it held the gas bubbling through the experience. At potentials higher than 0.75 V, the current drops slowly and this is due to the presence of hydrogen which collaborates with the formation of incipient hydrated oxide [15] , which takes place by the adsorption of OH groups. At higher potentials, the platinum oxide formed during direct cycle is not completely reduced and for this reason, the hydrogen oxidation current is lower in the reverse cycles than that in the direct cycles. For potential lower than 1.1 V, the reverse current cycle is the same as in direct cycles, indicating that the OH groups were removed from the surface at potentials lesser than 1.1 V in the reverse cycle. On the gold, (Figure 4), the metal oxide formation catalyzes the reaction and then the current is higher in

Figure 1. Plot of the maximum current of hydrogen oxidation in function of the square root of the potential sweep rate. Platinum electrode. H partial pressure: 0.1 atm, t = 25˚C.

Figure 2. Representation of hydrogen limiting current as a function of its partial pressure. The straight line is the linear fit of the experimental points set. Platinum electrode.
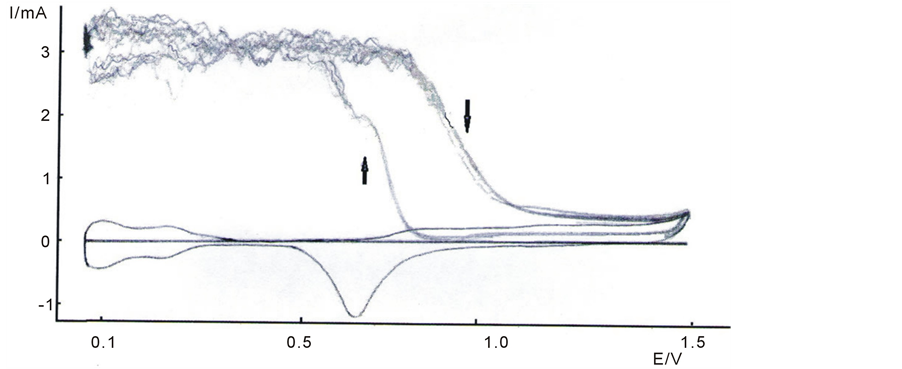
Figure 3. Voltammetric records obtained with platinum electrode in perchloric acid saturated with hydrogen gas, sweepning between 0.075 and 1.5 V; v = 0.1 V/s, t = 25˚C.
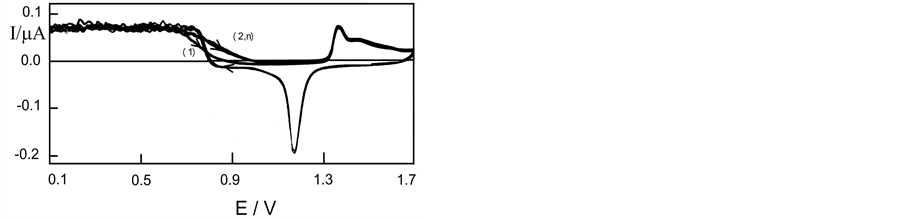
Figure 4. Voltammetric records obtained with gold electrode in perchloric acid saturated with hydrogen gas, sweepning between 0.1 and 1.7 V, v = 0.1 V/s, t = 25˚C.
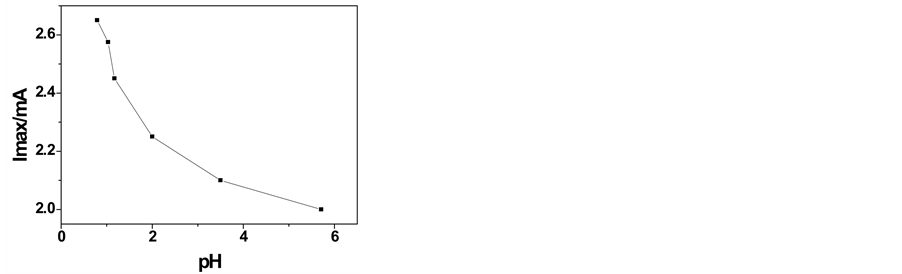
Figure 5. Plot of the H limiting current vs. pH. Platinum electrode. T = 25˚C.
the reverse cycle than that in the direct cycle.
At higher pH, the reaction current is lower, as shown in the Figure 5. The lowering the current as pH is increased has been observed also in previous works [2] and it shows the participation of hydrogen ions in the oxidation reaction.
4. Conclusions
When the partial pressure is lower than 0.1 atm, the H reaction is diffusion controlled on both materials.
In addition, the reaction is strongly shifted towards the right. The current drops abruptly when the surface reconstruction of plane (100) on gold lifts. On platinum, however, the current drop coincides with the formation of metal oxide at 0.8 V. Above this potential the contribution of both the reaction under study and the formation of the different metal oxides through the OH groups adsorbed is evident and the H oxidation is inhibited. On the other hand, sweeping the gold oxide potential region, the gold offers a more active surface for the H oxidation reaction.
The presence of H promotes the formation of both the hydrous platinum oxide and other metals oxides at higher potentials.
It is believed that under the experimental conditions the control of the hydrogen electrooxidation is diffusional. However, at higher potentials, the reactive contributes to the formation of different metal oxides through the adsorbed OH groups on platinum and the reaction is passivated.
Acknowledgements
The Universidad Nacional de San Luis supports this research. M.G. Sustersic is member of Consejo Nacional de Investigaciones Científicas y Técnicas. Jorge Zerbino is member of Comisión de Investigaciones Científicas de la Provincia de Buenos Aires.