Effects of flooding on arbuscular mycorrhizal colonization and root-nodule formation in different roots of soybeans ()
1. INTRODUCTION
Excess soil water generally inhibits the growth and nutrient uptake of soybeans (Glycine max (L.) Merril), and often reduce seed yields [1-3]. In several countries in monsoon Asia, such as Japan and Korea, soybean crops are cultivated in upland fields that have been converted from rice paddies. In such fields, excess soil water conditions during the rainy season frequently extensively damage this crop. There have been many reports of flooding injury in soybeans, such as leaf yellowing followed by lower photosynthesis and reduced root growth followed by lower nutrient uptake. These damages are induced through the lack of oxygen in the root system.
A typical adaptation to hypoxia in soybean plants is secondary aerenchyma formation, which functions to protect tissues during flooding [4-7]. Phellogen derived from the pericycle, which is the secondary meristem, develops into spongy tissues, and then forms an air space as the aerenchyma surrounding the stele. Aspects of root distribution to deeper soil layers under flooding conditions have also been described in soybean varieties such as Tachinagaha and En1282 [8]. Information on morphological responses to hypoxia is needed to improve flooding tolerance in this plant species.
Soybeans form symbioses with arbuscular mycorrhizal (AM) fungi and rhizobia (Bradyrhizobium japonicum), and the symbiotic nutrient acquisition controls the growth and seed yield of this crop grown under flooding. Most previous reports on the interactions among soybeans, AM fungi, and rhizobia have mainly focused on cultivation in upland fields [9-15]. On the other hand, there has been little information on their interactions in paddyrotated upland fields. Understanding the colonization of AM fungi and root nodule formation on soybean roots, therefore, is needed to improve the tolerance of soybean crops grown under flooded conditions, especially in Asian monsoon countries. In the present experiment, we investigated the effect of flooding during the early growth stage of soybeans grown in pots on AM colonization and root nodule formation in primary and lateral roots and adventitious roots.
2. MATERIALS AND METHODS
The pot experiment was conducted in a growth chamber (80 µmol∙m−2∙s−1, 25˚C/18˚C, day/night). Seeds of soybean cv. Fukuyutaka were sown in 1/10,000-are Wagner pots filled with granular soil for rice nurseries containing no fertilizer (Green soil; Izumo Green Epoch K.K., Japan). One gram of compound fertilizer (N:P2O5: K2O = 3:10:10) was applied to the pot prior to sowing. Five grams of AMF inoculums containing approximately 160 spores, mainly Gigaspora margarita CGC1411 (Central Glass Co. Ltd, Tokyo, Japan), were inoculated into the pot.
After the unfolding of the unifoliolate leaves on the first node (20 days after sowing), pots were divided into two groups. Seven pots were flooded (flooding), and the other 7 pots were left unflooded (irrigation). In the flooding treatment, the water level was maintained at 1 cm above the soil surface by adding water every 3 to 4 days. In the irrigation treatment, water was applied as needed.
After 39 days of flooding, four plants in each treatment were sampled and then separated into leaves, stems and petioles, primary and lateral roots, and adventitious roots emerging from the submerged part of the hypocotyl. Segments of the roots, which were separated into primary and lateral roots and adventitious roots, were taken and transverse sections with 3 replicates per segment were prepared to investigate aerenchyma formation. Following Mochizuki et al. [16], the sections were stained using 1% toluidine blue, and then the cross-sectional areas of the aerenchymatous tissue and stele were measured. The area of aerenchyma per section was measured using graphic editing software (GIMP 2.6.12) and the ratio of the aerenchyma area to the stele area was calculated.
The lengths of the roots, which were separated into primary and lateral roots and adventitious roots, were measured using the image analysis system WinRhizo (Regent Instruments Incorporated, Canada). After measuring root length, a portion of the roots was sampled to determine the AM colonization of the roots. To quantify colonization, the percentages of root segments containing internal hyphae and arbuscules were determined using a grid measuring method [17]. Root nodules attached on the primary and lateral roots and adventitious roots were separately counted. Leaves, stems and petioles, primary and lateral roots, and adventitious roots were oven-dried at 70˚C and then weighed.
Results are expressed as means ± standard deviation of mean. The statistical difference was determined by Student’s t-test. Statistical analysis was performed using Excel Tokei 2008 software version 1.05 (SSRI Co Ltd, Tokyo, Japan).
3. RESULTS AND DISCUSSION
The fourth trifoliate leaves at the third node of the main stem had emerged in both treatments at 39 days after flooding. Typical morphological alterations in submerged hypocotyls on the soil surface, such as an enlarged diameter and partial cracking of the surface tissues, were found in the flooding treatment (Figure 1). Adventitious roots developed on the soil surface were also characterized as a response to the flooding. These responses were not observed in the irrigation treatment. Previous research has shown that flooding to soybean plants generally causes these phenomena [4-7].
The dry weights of leaves and of stems and petioles were not significantly different between the two treatments (Table 1). The dry weight of primary and lateral roots in the flooding treatment was significantly lower than that in the irrigation treatment and that of adventitious roots in the flooding treatment was conversely greater than in the irrigation treatment, indicating that the response to flooding during the early growth stage could be definitely characterized by both vigorous growth of adventitious roots and inhibition of primary and lateral roots. Although total root length was not different between the two treatments, the length of primary and lateral roots was shorter in the flooding treatment, and that of adventitious roots was longer than in the irrigation treatment (Table 2). Adventitious roots comprised about 90% of the total root length in the flooding treatment. As a morphological adaptation to flooding, the rapid emergence of adventitious roots has also been reported in many plant species such as Sesbania [18], Pterocarpus officinalis Jacq. [19], and azuki beans [20]. These roots were connected by an aerobic environment, improving the aeration of belowground parts [21,22]. In addition to the aerenchymatous tissues formed after flooding described below, these roots also transport oxygen from the atmosphere to the rhizosphere [6].
Considering these responses, we defined the aerenchyma formation of the adventitious roots and also measured the AM colonization and root nodule formation in soybeans grown under flooded conditions. The ratio of the aerenchyma area to the stele area was 82.5% in adventitious roots (Table 2). In contrast, those tissues were not observed in the irrigation treatment, suggesting that these tissues of adventitious roots might influence the AM colonization.
In the irrigation treatment, the AM colonization ratio was 12.5% in the primary and lateral roots and 14.5% in

Table 1. Effect of flooding on dry weight (g) of soybean grown in pot for 59 days after sowing.

Table 2. Effect of flooding on root length and aerenchyma formation of soybean grown in pot for 59 days after sowing.
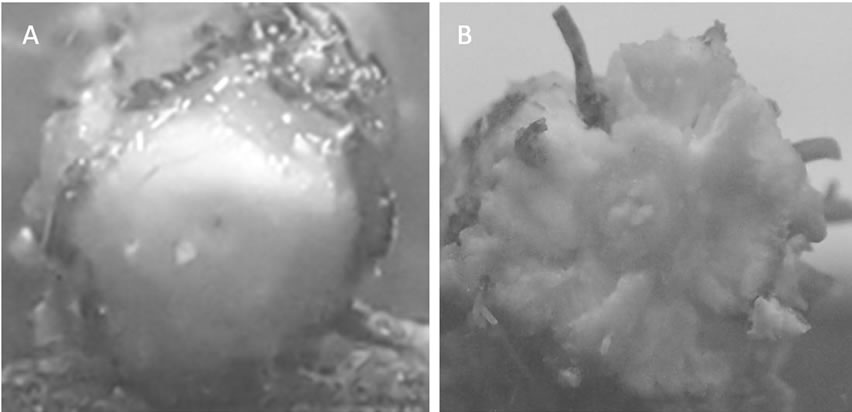
Figure 1. Aerenchymatous tissues formation in soybean seedlings after flooding. A: Irrigation, B: Flooding.
the adventitious roots (Table 3). The ratios in the flooding treatment were significantly less than those in the irrigation treatment. On the other hand, the AM colonization ratio in the flooding treatment was markedly low in the primary and lateral roots (0.8%), while it was not necessarily low in the adventitious roots (7.5%).
Most root nodules were formed not on the primary and lateral roots but on the adventitious roots, especially in the flooding treatment (Table 4). However, there was no significant difference in the number of root nodules on the adventitious roots and the total number of nodules between the two treatments, because the values had con-

Table 3. Effect of flooding on AM colonization ratio (%) into the roots of soybean grown in pot for 59 days after sowing.

Table 4. Effect of flooding on number of root nodules of soybean grown in pot for 59 days after sowing.
siderable variation among plants, especially in the flooding treatment.
In this experiment, AM colonization and root nodule formation on the two different types of roots were detected, that is, the root system was separated into primary and lateral roots as basal roots, and adventitious roots emerged below the lower position of the hypocotyl. In the flooding treatment, the latter were distributed on the soil surface and in the shallow soil layer in the pot. Hence, the roots might be not damaged by lower oxygen conditions. In fact, the roots had a higher AM colonization ratio and many root nodules were formed. According to Lynch [23], the adventitious roots might have a greater abundance of aerenchyma than other root types. In this study, the ratio of the aerenchyma area to the stele area in the roots was 82.5% in the flooding treatment. Although the high rates of AM infection and nodule formation in the adventitious roots induced in the flooding treatment might be predictable because the microorganisms are aerobic [24,25], the findings help understanding the response to flooding in soybeans and to clarify the different rates between the two different types of the roots. Further studies should examine whether the internal hyphae of AM fungi infecting aerenchymatous roots elongate beside the porosity and also how rhizobia can infect the roots with a higher rate of porosity.
4. ACKNOWLEDGEMENTS
This study was supported in part by MEXT, KAKENHI, Japan (No. 24658021, 24380012).