1. Introduction
Hydrogen generation from biomass-derived alcohols has been the activity of choice recently. Ethanol is more attractive because it is non-toxic, has higher hydrogen content, is renewable energy and has an easy-to-handle nature when compared to methanol [1,2]. The main catalytic reaction using ethanol to produce hydrogen by steam reforming is shown in Equation (1), where only hydrogen and non-renewable CO2 are produced, providing 6 moles of H2 per mole of ethanol stoichiometrically [3].
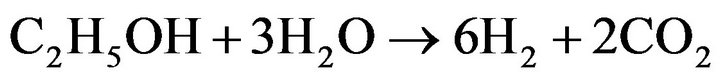 (1)
(1)
Several metallic active phases have been used as catalysts for the steam reforming of ethanol (SRE) to produce hydrogen. Since Co-based catalysts, mainly metal exhibiting appreciable activity for C-C bond broken and water-gas shift (WGS) reactions, generate a low temperature and few by-products, they are efficient when used in SRE. The early stage of SRE research has focused on Co-based catalysts. Haga et al. [4] found that the properties of cobalt catalysts were greatly influenced by the supports, where the hydrogen production decreased in the order of: Co/Al2O3 > Co/ZrO2 > Co/MgO > Co/SiO2 > Co/C. The Co/Al2O3 catalyst showed high hydrogen selectivity for SRE by suppressing CO methanation and ethanol decomposition. Supported cobalt catalysts showed a significant improvement in catalytic performance on the SRE compared with corresponding supports reported by Llorca et al. [5], a variety of oxides involving acidic/ basic and redox properties. Batista et al. [6] studied the high efficiency SRE over Co/Al2O3 and Co/SiO2 catalysts with little Co content (8%) in which the Co/SiO2 catalyst showed better CO removal. Llorca et al. [7] reported CO-free hydrogen produced from SRE over the Co/ZnO catalyst at low temperatures, where the highly stable catalyst was prepared by using Co2(CO)8 as a precursor.
The technique of doping extra components, such as alkali (Li, Na and K) [8], alkaline earth (Mg and Ca) [9, 10] and lanthanide (La and Ce) [10] to modify the originnal property and improve the performance of a catalyst is interesting. Pigos et al. [8] reported that the addition of Na and K significantly improved the formate decomposition rate on a WGS reaction over Pt/ZrO2 catalysts. Wang et al. [9] reported that the addition of Na improved the catalytic performance of a PtRu/ZrO2 catalyst on the oxidative steam reforming of ethanol, where the Na not only enhanced the WGS reaction at a low temperature, but also reduced coke deposition. Cheng et al. [10] also reported the promotional effect of doping alkaline earth oxides or lanthanide oxides on a Ni/Al2O3 catalyst for CO2 reforming of CH4.
Besides the selection of an active metal or promoter for the supported catalysts, the choice of a support with a high surface area to disperse the metal phase over their surface is a main consideration to enhance catalytic performance. Support material, such as γ-Al2O3, SiO2, ZSM-5 [11], MCM-41 [12] and SBA-15 [13], have been widely used in recent years as catalyst supports for catalytic reactions occurring at high temperatures, based on the support material’s larger pores, thicker walls and higher thermal stability. Of considerable interest in this regard are mesoporous materials as a support that will provide an improvement on hydrogen production via steam reforming reaction [14-19]. The promoter effect of alkaline earth metals (Mg and Ca) over Cu-Ni/SBA-15 [16] and Cu-Ni/SiO2 [18] catalysts has been studied; both of them improved the dispersion of the metallic phase and strengthened the metal-support interaction. High hydrogen selectivity was obtained with Mg and reduced deposited carbon with the incorporation of Ca. A promoter made up of a CexZr1−xO2 layer pre-coated on SBA-15 changes the redox properties and enhances the catalytic activity on steam reforming of methane over a Ni-based catalyst, as reported by Wang et al. [19].
It is well known that Co-based catalysts suffer from deactivation by carbon deposition at high reaction temperatures [20]. This is obviously an important point to consider in SRE reactions related to Co-based catalysts. The SBA-15 supported Co catalysts with high surface area and modified by an Mg promoter were prepared in this work. The catalytic performance and coking behavior of hydrogen production via SRE over mesoporous structure catalysts were also considered.
2. Experimental
2.1. Catalyst Preparation
SBA-15 was prepared according to the method described in the literature [13]. Briefly, a triblock copolymer P123 (8 g, Strem) was dissolved in 250 mL HCl (1.9 M). The solution was stirred at 40˚C for 2 h, and 16 g of tetraethyl orthosilicate (TEOS) were then slowly added to the mixture and stirred vigorously at 40˚C for 22 h. The solution was transferred into a Teflon bottle and aged at 100˚C for 24 h. The solid product was filtered, washed with deionized water and then dried at room temperature for 24 h, followed by calcination in air at 500˚C for 6 h with a heating rate of 7˚C /min.
Catalysts promoted with alkaline are much more sensitive to the preparation order for catalytic performance, and the promoting effect is more significant when the support is impregnated with the promoter oxides before the incorporation of the active phase [10]. For this reason, Mg-modified Co/SBA-15 catalysts are prepared by consecutive impregnation with Mg and then Co. Mgx/SBA- 15 samples were prepared from the aqueous solution of Mg(NO3)2·6H2O (Mg loading, x = 5 and 10 wt%, Showa) incorporating SBA-15 by the impregnation method. CoyMgx/SBA-15 samples were prepared by the incipient wetness impregnation method using Mgx/SBA-15 with aqueous Co(NO3)2·6H2O (Co loading, y = 10 and 20 wt%, Showa). All samples were dried at 100˚C overnight and then calcined at 300˚C for 3 h.
2.2. Catalyst Characterization
The metal loading of catalysts was determined by the atomic-emission technique (ICP-AES) using a Perkin Elmer Optima 3000 DV. The BET surface area and pore size distribution were measured by N2 adsorption at a liquid nitrogen temperature using a Micromeritics ASAP 2010 analyzer. X-ray diffraction (XRD) measurement was performed using a Siemens D5000 diffractometer with Cu Kα1 radiation (λ = 1.5406 Å) at 40 kV and 30 mA. The microstructure and particle size of the samples were observed by using transmission electron microscopy (TEM) with a JEOL JEM-2010 microscope equipped with a field emission electron source and operated at 200 kV. Reduction behavior of CoyMgx/SBA-15 catalysts was studied by temperature-programmed reduction (TPR). About 50 mg of the sample were heated in a flow of 10% H2/N2 gas at a flow rate of 10 ml∙min−1. During TPR, the temperature was increased by 7˚C∙min−1 from room temperature to 900˚C.
2.3. Activity Tests
Catalytic activity of CoyMgx/SBA-15 catalysts in an SRE reaction was determined at atmospheric pressure in a fixed-bed flow reactor. 100 mg of the catalyst were placed in a 4 mm i.d. quartz tubular reactor and held by glass-wool plugs. The temperature of the reactor was controlled by heating tape and measured by a thermocouple (1.2 mm i.d.) at the center of the reactor bed. The feed of the reactants was comprised of a gaseous mixture of ethanol (EtOH), H2O and Ar (purity 99.9995%, supplied by a mass flow controller). The composition of the reactant mixture (H2O/EtOH/Ar = 37/3/60 vol%) was controlled by the Ar flow stream (22 mL/min) through a saturator (maintained at 120˚C) containing EtOH and H2O. The gas hourly space velocity (GHSV) was maintained at 22,000 h−1 and the H2O/EtOH molar ratio was 13 (H2O:EtOH = 80:20 by volume). Prior to reactivity measurement, the catalyst was reduced in 10% H2 in N2 for 2 h at 400˚C. The SRE activity was tested stepwise, increasing the temperature from 350˚C to 550˚C. The reaction was carried out online by gas chromatography (GC) with columns of Porapak Q (for CO2, H2O, C2H4, CH3CHO, CH3OCH3 and EtOH) and using a Molecular Sieve 5 Å (for H2, CH4 and CO) for separation. It was also quantitatively analyzed by two sets of thermal conductivity detectors (TCD) on line. Response factors for all products were obtained, and the system was calibrated with appropriate standards before each catalytic test. Activity evaluation of all samples depended on the conversion of ethanol (XEtOH), the distribution of products (mol %) and the yield of hydrogen (YH2, mol H2/mol EtOH) according to the following equations.
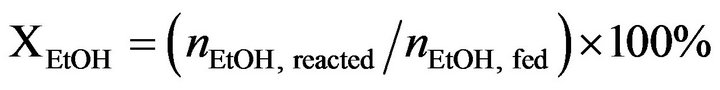 (2)
(2)
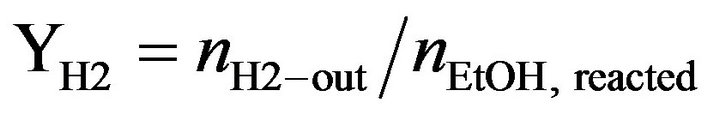 (3)
(3)
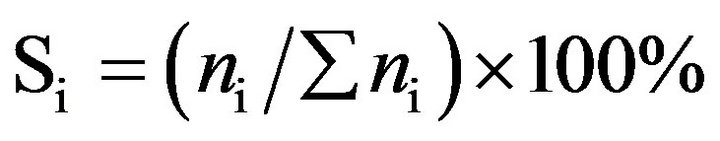 (4)
(4)
where ni was a mole of products and included H2.
3. Results and Discussion
3.1. Characterization of Supports and Catalysts
The XRD patterns at small angles of SBA-15, Mgx/ SBA-15 and CoyMgx/SBA-15 (x = 5 and 10; y = 10 and 20) samples are shown in Figure 1. The SBA-15 support (Figure 1(a)) shows a pattern with three well-resolved peaks observed at 2θ values of 0.92˚, 1.54˚ and 1.77˚ that correspond to the diffraction of (100), (110) and (200) planes, respectively, indicating their ordered 2D hexagonal structure with space group p6mm [13]. The d-spacing of this structure, calculated from nλ = 2dsinθ is 9.6 nm, which is also in the mesoporous range. Both Mgx/ SBA-15 samples (x = 5 and 10) are presented in Figures 1(b) and (c), respectively. The intensity of the diffraction peaks of the hexagonal mesostructure decreases gradually with the increase of x from 5 to 10. Moreover, a similar trend can be observed with the decrease in d-spacing where the d-spacing for x = 5 and 10 are 9.3 and 9.0 nm, respectively. The intensity of diffraction peaks for the CoyMgx/SBA-15 (y = 10 and 20) catalysts (Figures 1(d)-(h)) decreases with the increase of x and y, and weakens more than the Mgx/SBA-15 samples. Furthermore, the material composed of a high surface area, larger pores and thicker walls seems to disintegrate with increasing metal loading, raising doubt about the structural integrity.
The N2 adsorption-desorption analysis of the CoyMgx/ SBA-15 catalysts is shown in Figure 2. All of the samples exhibit a Type IV isotherm with a clear H1-type
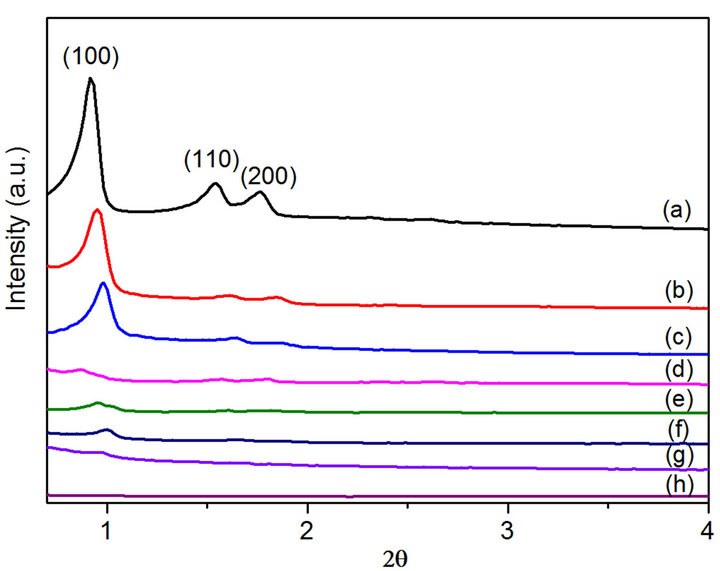
Figure 1. Small angle XRD patterns of the samples: (a) SBA-15 (b) Mg5/SBA-15 (c) Mg10/SBA-15 (d) Co10Mg5/ SBA-15 (e) Co20Mg5/SBA-15 (f) Co20Mg5/SBA-15-H650 (g) Co10Mg10/SBA-15 (h) Co20Mg10/SBA-15.
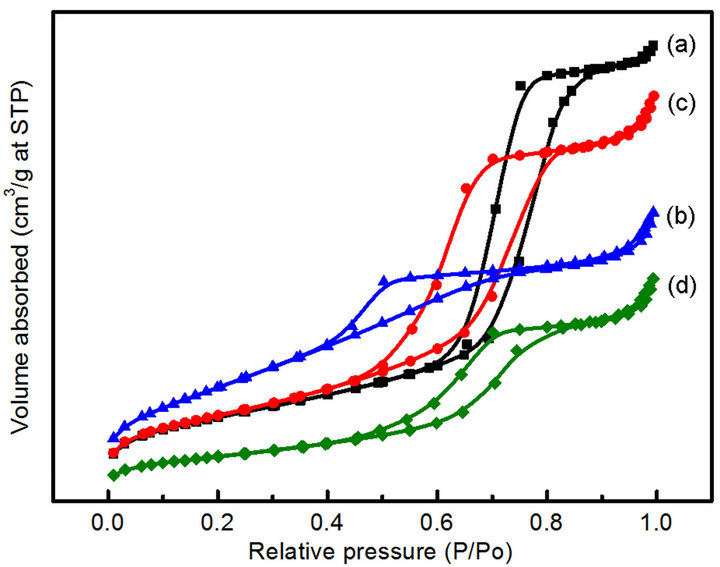
Figure 2. N2 adsorption/desorption isotherms of the samples: (a) Co10Mg5/SBA-15 (b) Co10Mg10/SBA-15 (c) Co20Mg5/ SBA-15 (d) Co20Mg10/SBA-15.
hysteresis loop, with metal loading or not (SBA-15 and Mgx/SBA-15 samples are not shown), which is typical for mesoporous materials. Even though the XRD analysis showed the destruction of the hexagonal structure with impregnation of cobalt, the SBA-15 supported catalysts still maintained the mesoporous structure. Table 1 summarizes the physical characterization of CoyMgx/SBA-15 catalysts, which includes the metal loading, surface area and phase composition. The surface area decreases with the increase of the (x + y) value, where the surface areas are 359, 313, 234 and 130 m2/g, respectively for the values of 15, 20, 25 and 30. The decrease of surface area indicates that the mesoporous structure may be blocked by large amounts of Mg and Co loading.
The wide-angle XRD patterns of the CoyMgx/SBA-15 catalysts are shown in Figure 3. The broad and wide

Table 1. Physical characterization of the CoyMgx/SBA-15 catalysts.
peak at 2θ around 15˚ - 30˚ is characteristic of amorphous silica. The peak related to MgO (2θ ≈ 42˚) is unobservable in the XRD patterns for CoyMgx/SBA-15 catalysts, which indicate the Mg shows highly dispersed on SBA-15 or becomes the nickel-magnesia solid solution oxides (Co, Mg)O [21,22]. Both the Co10Mg5/ SBA-15 and Co10Mg10/SBA-15 catalysts (Figure 3(a) and (b)) show the characteristic diffraction peaks corresponding to the (220), (311), (511) and (440) planes at 31.3˚, 36.8˚, 59.0˚ and 64.8˚, respectively. These are related to the cubic phase of Co3O4 (JCPDS No: 76-1802). The spinel structure of magnesium cobaltite MgCo2O4 [23,24] (JCPDS No: 81-0671) shows the corresponding planes of (111), (220), (311), (400), (511) and (440) at 18.9˚, 31.1˚, 36.6˚, 44.5˚, 58.9˚ and 64.7˚, respectively. These are obtained on the high Co loading catalysts of Co20Mg5/SBA-15 and Co20Mg10/SBA-15 (Figures 3(c) and (d)). Otherwise, the higher Co loading would show the stronger diffraction signal. In here, both the Co3O4 and MgCo2O4 phases are not able to give clear assignment, because their diffraction peaks are overlapped. Choudhary et al. [25] reported that the MgCo2O4 phase was only observed in the case of catalysts with high Co loadings, such as over 20%, which was supported by our results when y = 20. Therefore, the CoyMgx/SBA-15 catalysts may contain two phases of Co3O4 and MgCo2O4, and further investigation will be discussed on TPR analysis.
Figure 4 shows the TPR profiles of the CoyMgx/SBA- 15 catalysts. There are two continuous reduction peaks around 180˚C to 350˚C and broad peak around 500˚C to 700˚C, respectively. While the lower temperature peaks may be related to the two-steps reduction of Co3O4 [26] and the higher temperature peak is assigned the reduction of MgCo2O4 [25]. Besides, a faint peak over 800˚C may be attributed to the reduction of cobalt-magnesia solid solution oxides (Co, Mg)O formed on the catalysts [27]. Further, the reduction signal of Co3O4 would be raised by increasing the Co loading. These results are confirmed to the XRD study, the Co3O4 and MgCo2O4 phases are coexisting in CoyMgx/SBA-15 catalysts. Particularly, the