Factors Research on the Influence of Leaching Rate of Nickel and Cobalt from Waste Superalloys with Sulfuric Acid ()
1. Introduction
Ni and Co have excellent physical and chemical properties and mechanical properties, such as high temperature resistance, corrosion resistance, high strength and strong magnetism etc. The production of new materials [1], especially superalloys, play a key role in aviation, aerospace, and other relevant departments of industry. With the development of aerospace career, the demand of these new materials is increasing rapidly, i.e., the requirement of nickel and cobalt are also keeping increasing. However, the shortage of nickel and cobalt mineral resource is becoming more and more serious, secondary recovery of waste superalloys was thus put forward and studied in many countries to avoid wasting of the recyclable resources [2]. At present, the main extraction methods of cobalt and nickel include: acid leaching and high pressure acid leaching with sulfuric acid, hydrochloric acid, nitric acid, or ammonia leaching, chlorine leaching [3-12], and sulfide precipitation [13,14], electrolytic deposition method [15,16], and the carbothermal reduction [17], acid leaching following roasting [18] with hydrometallurgical process or pyrometallurgical process or pyro-hydro-metallurgical process. These processes are mainly aimed at treating various kinds of raw ore, waste ion battery, waste catalyst materials, relatively rare to treat waste superalloys materials. In this paper, after melting and milling the waste superalloy scrap, the authors investigated leaching of nickel and cobalt from waste superalloys with sulfuric acid directly, the influence factors on the leaching rates of nickel and cobalt ware mainly discussed based one the experimental results.
2. Experimental
2.1. Experimental Materials
The materials used in this experiment were prepared by air-atomization. The average compositions of waste superalloys are listed in Table 1.which were determined with the methods of XRF, titration or FAAS. The SEM images in the Figure 1 shows the micrograph of waste superalloys. Figure 2 shows the waste superalloys mainly contain solid solution of Ni, Co, Al, Mo, Ta etc. as can be seen from the X-ray pattern shown in Figure 2. Acid insoluble components are mainly W, Mo, Ta etc.
 (a)
(a) (b)
(b)
Figure 1. (a) The SEM images of waste superalloys powders; (b) Optical micrograph of waste superalloys (1. Ta, Nb, Ni; 2. Ni, Co, W, Al; 3. Ca, Si).
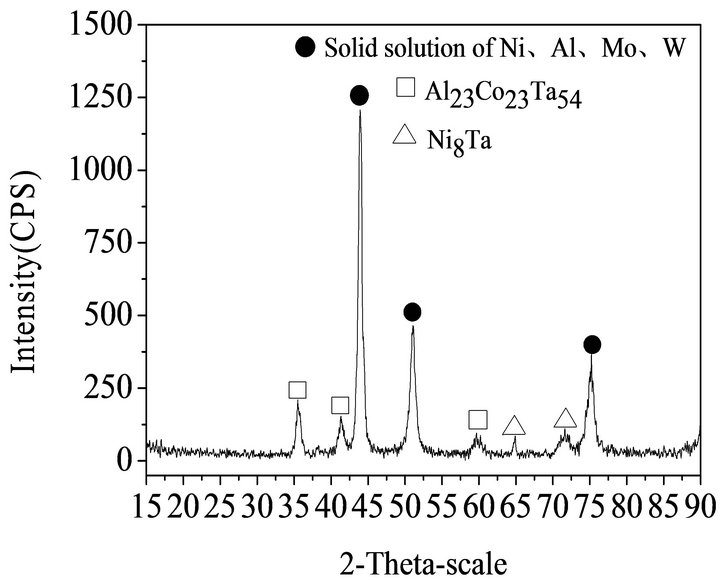
Figure 2. XRD pattern of waste superalloys.
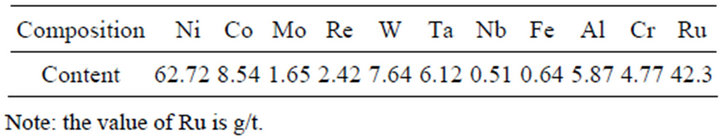
Table 1. Chemical compositions of waste superalloys (wt/%).
which were utilized in following process. The waste samples were crushed and screened under 80 - 120 mesh.
2.2. Experimental Methods
2.2.1. Experimental Process
The waste superalloys were melted at 1400˚C, and then made into powders with gas atomization. The powders were milled into different sizes for leaching with sulfide acid. The experimental flow chart of the leaching of Ni and Co from waste superalloys was shown in Figure 3.
2.2.2. Sulfide Leaching Tests
Sulfide leaching tests were carried out in 900 mL beakers. Add sulfide acid into the beaker and heat to a given temperature, then add the measured waste superalloys powders with mechanical stirring. During the reaction, constantly adding water to keep the volume unchanged. After the reaction, the volume of filtrate was measured, and the contents of Ni and Co in filter residue were analyzed.
2.2.3. Experimental Principle
Considering the waste superalloys contain Ni, Co and Fe, the tests were performed with sulfide acid as Ni, Co and Fe could be dissolved by sulfide acid, producing sulfate and H2. During the leaching, the main reactions were considered as follows:
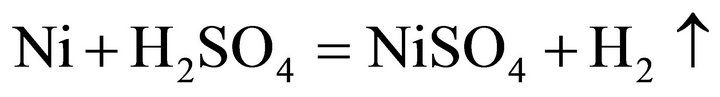 (1)
(1)
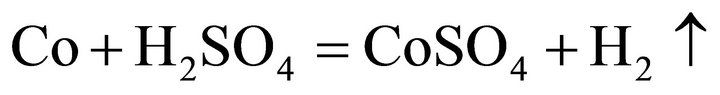 (2)
(2)
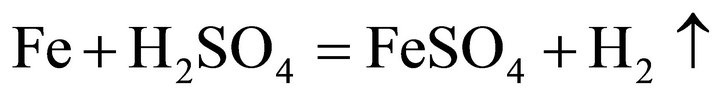 (3)
(3)
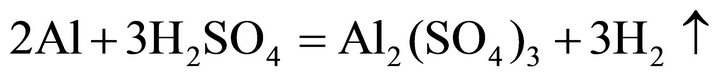 (4)
(4)
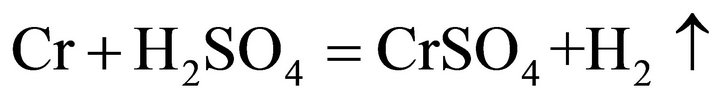 (5)
(5)
3. Results and Discussion
3.1. Effect of the Temperature on Leaching Rate of Nickel and Cobalt
The effect of temperature on leaching rate of Ni and Co can be seen in Figure 4" target="_self"> Figure 4 and the leaching time is fixed as 5 hours, the sulfide concentration is fixed as 40 wt%, the stirring speed is fixed as 250 r/min, the size of the powder is fixed as −80 + 120 mesh. It can be seen in Figure 4 that the leaching rate of Ni and Co is influenced greatly by reaction temperature, the leaching rate of Ni and Co

Figure 3. Flow chart of leaching Ni, Co from waste superalloys.
is as much as 96.68 wt% when temperature increased to 85˚C. Although the leaching rate can be improved slightly with increasing temperature, it is difficult to keep the temperature increasing as the energy will increase simultaneously. Therefore, the optimum temperature is 85˚C.
3.2. Effect of the Leaching Time on Leaching Rates of Nickel and Cobalt
Figure 5 shows the effect of leaching time on leaching rates of Ni and Co with other conditions are fixed. Figure 5 shows that at the beginning of leaching, the leaching rate of Ni and Co increase very quickly when leaching time increased to 5 hours, the leaching rate of Ni and Co can achieve above 96%, and then although with the leaching time increasing, the leaching rate increased slowly. Considering the efficiency and energy, 5 hours was chosen as the optimum leaching time.
Figure 6 shows that the peaks intensity of solid solution of Ni (containing Co, Al etc.) are decreasing with the time increasing, and some new different complicate chemical phase began to appear.
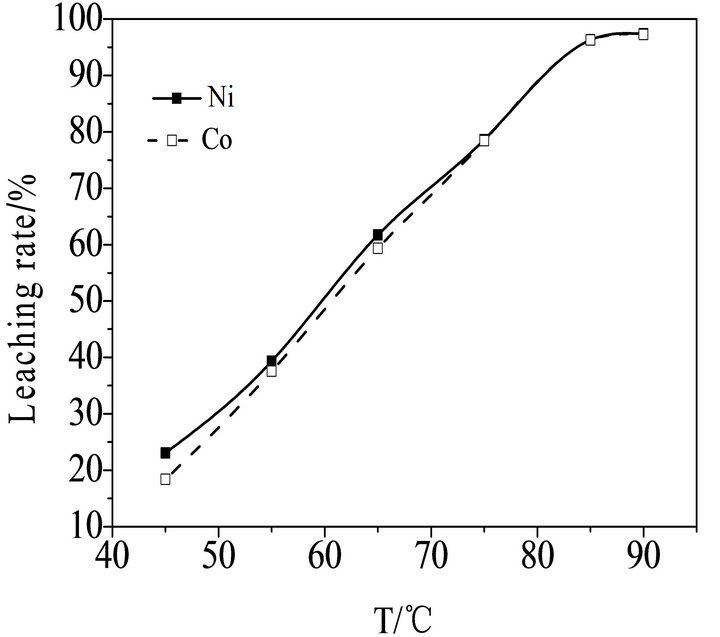
Figure 4. Effect of the temperature on leaching rate of nickel and cobalt.
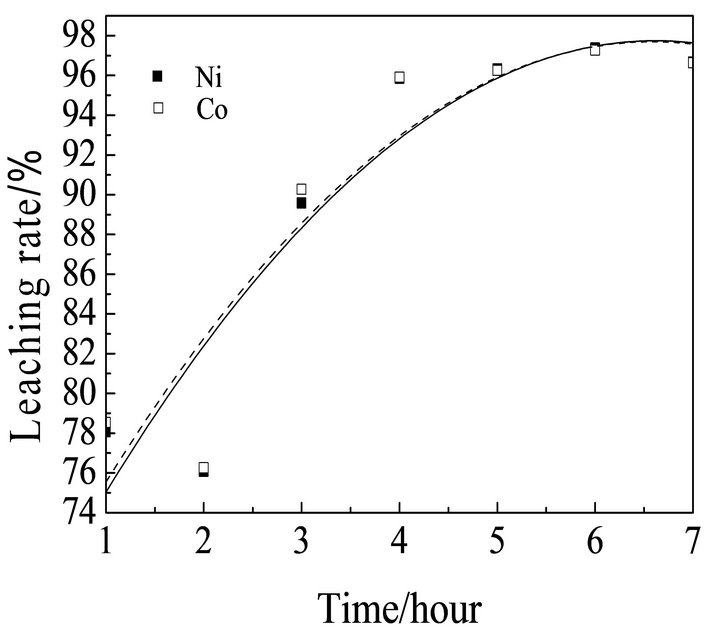
Figure 5. Effect of the leaching time on leaching rate of nickel and cobalt.
3.3. Effect of the Sulfuric Acid Concentration on Leaching Rates of Nickel and Cobalt
The effects of sulfuric acid concentration on leaching rates of Ni and Co were shown in Figure 7 with other conditions were fixed. Figure 7 shows that with increasing of sulfuric acid concentration, the leaching rate of Ni and Co increase obviously. When the sulfuric acid concentration increased to 40 wt%, the leaching rate are above 96%, while it can be seen that the increasing is relatively slow. Therefore the optimum sulfuric acid concentration is determined as 40 wt%.
3.4. Effect of the Stirring Speed on Leaching Rates of Nickel and Cobalt
Figure 8 shows that the effects of stirring speed on leaching rates of Ni and Co with other conditions are fixed. It can be obviously seen that, the leaching rate increase greatly initially with increasing the stirring speed. When the stirring speed increased to 250 r/min, the leaching rate of Ni and Co can achieve a satisfied result. But when the speed is increased again, the leaching rate of Ni and Co decreases greatly. Because the materials will be rotating with the solution, the effect of stirring is decreased.