Correlation between Intestinal Health and Coccidiosis Prevalence in Broilers during Different Seasons of the Year in Brazil from 2012 to 2018 ()
1. Introduction
In 2020, Brazil held the top position as the largest exporter of chicken meat, with a total export volume of 13.845 million tons. Furthermore, the average per capita consumption of chicken meat in Brazil was reported to be 45.27 kg per person. To maintain optimal productivity in this setting, it is crucial to efficiently manage infections that pose a threat to the poultry industry, such as coccidiosis. This parasitic infection, caused by protozoa of the genus Eimeria spp., leads to significant losses due to illness and mortality. In 2016, it was estimated that this illness incurred annual costs worldwide ranging from $7 to $13 billion [1] .
The Eimeria species commonly found in poultry birds comprise Eimeria acervulina, Eimeria maxima, Eimeria brunetti, Eimeria mitis, Eimeria praecox, Eimeria necatrix, and Eimeria tenella. This pathogen undergoes intracellular development in specific regions of birds’ gastrointestinal tract, leading to reduced weight gain and increased feed conversion. This is due to severe damage to the intestines, resulting in increased intestinal permeability. Additionally, it induces excess mucus and fluid, intestinal desquamation, and erosion of the gizzard. Furthermore, coccidiosis disrupts intestinal energy metabolism by affecting ATP production and consumption, leading to inflammation and oxidative stress [2] . Consequently, when intestinal balance is disrupted, birds become more prone to other illnesses like Clostridium perfrigens. This impacts both the health and productivity of the birds [3] .
Due to Brazil’s vast geographical expanse and varied climates, it is imperative to fully comprehend how animal intestinal health relates to coccidiosis throughout different seasons. Understanding this relationship is crucial for developing diverse prevention strategies aimed at reducing the incidence of coccidiosis and mitigating its economic impact on the poultry industry.
However, there is limited and contradictory evidence regarding the occurrence of coccidiosis across various seasons. Razmi and Kalideri (2000) [4] noted a higher incidence of clinical and/or subclinical coccidiosis during the spring and winter seasons. In contrast, Ahad et al. (2014) [5] reported a higher incidence during the autumn and summer seasons. This study aimed to determine if there is a connection between seasonal variations and the presence of specific lesions induced by Eimeria spp. in the gastrointestinal tract of broilers in Brazil from 2012 to 2018.
2. Materials and Methods
2.1. Animals and Data Set
A study was undertaken to assess the intestinal health of broiler chickens across 103 poultry companies located in different states of Brazil from 2012 to 2018. The study involved a total of 8607 chickens from the Cobb and Aviagen lines, aged between 14 and 42 days. It is important to note that this study was purely observational. Consequently, the feed given to the birds originated from their respective integrated companies, with no involvement or direction from the evaluator concerning the content or utilization of growth promoters or anticoccidial drugs. Additionally, the evaluated broilers did not undergo any coccidiosis immunization programs. The data from all four seasons - spring, summer, autumn, and winter - was analyzed to determine the correlation between the presence of intestinal lesions and the different seasons.
2.2. Sampling
The assessment was conducted on a random selection of 3 to 6 avian specimens from each poultry barn, specifically at three distinct locations within the chicken building (entrance, middle, and exit). Euthanasia was conducted via cervical dislocation, in accordance with the animal welfare and euthanasia guidelines outlined by Brasil/MCTI in 2013 [6] . The macroscopic evaluation involved examining cellular desquamation, excessive fluid and mucus, ingestion of bedding and mealworms, alterations in intestinal thickness and tone, food passage, presence of worms (Raillietina spp.), duodenitis, enteritis, necrotic enteritis, and erosion of the gizzard [7] . Figure 1 depicts the occurrence of lesions induced by E. acervulina, E. maxima, and E. tenella in the gastrointestinal tract. Lesions were categorized based on their severity using the Johnson and Reid (1970) [8] methodology, with a score of 0 representing no injury and a score of 4 indicating severe damage.
Furthermore, the microscopic examination (at a magnification of 100×) evaluated the existence of E. maxima oocysts in the intestinal mucosa surrounding the Meckel’s diverticulum. This assessment was conducted at five specific locations on the slide, which included the four corners and the center. The microscopic scores were assessed on a scale of zero to four, where zero indicated the absence of oocysts, one indicated 1 to 10 oocysts, two indicated 11 to 20 oocysts, three indicated 21 to 40 oocysts, and four indicated more than 41 oocysts [9] , as depicted in Figure 2.
The species E. acervulina (18.3 × 14.6 µm), E. maxima (30.5 × 20.7 µm), and E. tenella (22 × 19 µm) were assessed according to a previous study conducted by [7] . The results can be seen in Figure 3.
2.3. Statistical Analysis
The normality test (Shapiro-Wilk) was applied to all variables. Subsequently, the data underwent analysis of variance (ANOVA) using SAS 9.3 software, and correlation tests between lesions and necropsy findings during the four seasons of the year. A difference between treatments was considered significant when P = 0.05.
3. Results
Characteristics such as cell desquamation, excessive fluid and mucus, ingestion of bedding and mealworms, intestinal thickness, food passage, intestinal tone, Turkish towel appearance, presence of worms (Raillietina spp.), duodenitis, enteritis, necrotic enteritis, and gizzard erosion were evaluated as described by [7] . Table 1 displays the relationships among intestinal changes during the several seasons of the year, namely Summer (S), Autumn (A), Winter (W), and Spring
![]()
Figure 1. Presence of lesions caused by E. acervulina, E. maxima, and E. tenella.
![]()
Figure 2. Four scoring categories were utilized for classifying Eimeria maxima micro assay.
![]()
Figure 3. Detection of oocysts for E. acervulina, E. maxima, and E. tenella.
Table 1. Correlation between broiler’s intestinal alterations traits and annual seasons.

Legend: S—Summer; A—Autumn; W—Winter; Sp—Spring.
(Sp). The statistical analyses for each season of the year are displayed in tables. Table 2 shows a significant correlation between broiler’s intestinal alterations parameters and summer season. Table 3 depicts a statistical correlation between broiler’s intestinal alterations traits and autumn season. In Table 4 we observed a significant correlation between broiler’s intestinal alterations traits and winter season. Whereas, Table 5 indicates a significant correlation between broiler’s intestinal alterations traits and spring season. The data considered in these analyses have a significance level of P = 0.05. Below, we will present the most notable findings obtained from the statistics presented in Tables 2-5. Food passage showed correlation with cell desquamation, excess mucus, and litter consumption across all four seasons (P = 0.05). During the spring season, there was a correlation between food transit and an abundance of fluid (P = 0.0167), as well as the presence of both thick (P = 0.0375) and thin (P = 0.0061) intestines. The winter and fall seasons exhibited a correlation with reduced intestinal thickness (P = 0.0051 and P = 0.0009, respectively), but the summer season showed a correlation with increased intestinal fluid levels (P = 0.0001). Throughout all seasons of the year, there was a clear positive relationship between E. acervulina and intestinal desquamation, excess mucus, bedding intake, food passage, and Turkish towel appearance (P = 0.05).
During autumn, winter, and spring, E. acervulina was found to have a significant association with duodenitis and overall enteritis (P = 0.05). Moreover, it is linked to the presence of worms throughout autumn (P = 0.0465).
There was a significant correlation between food intake and the shedding of cells, excessive production of mucus, and consumption of litter throughout the four seasons (P = 0.05).
E. maxima was consistently linked to intestinal scaling, elevated fluid levels, and mucus production throughout all four seasons (P = 0.05). Eimeria maxima had a significant correlation with bedding intake, food passage and Turkish towel appearance, while Eimeria acervulina only during the summer, winter, and spring seasons (P = 0.05). It is important to highlight that E. maxima is the sole species that is not associated with worm presence throughout any of the seasons.
Eimeria tenella had a significant correlation with increased mucus production during all four seasons (P = 0.05). Additionally, it was associated with inflammation of the duodenum and small intestine during the summer, winter, and spring (P = 0.05). Furthermore, there is a correlation between the existence of worms and this phenomenon (P = 0.0154). Additionally, there was a significant positive correlation between E. tenella and E. acervulina during the winter (P = 0.0018) and spring (P = 0.0047). Nevertheless, it is associated with E. maxima during the autumn (P = 0.001) and spring (P = 0.0409).
During the spring season, there was a significant correlation between E. maxima micro and E. maxima (P = 0.0010) as well as E. tenella (P = 0.0036). The association with E. acervulina is observed during the winter (P = 0.0162) and spring (P = 0.0001). E. maxima micro is linked to increased clinical signs in the spring, such as shedding of cells, excessive fluid and mucus, ingestion of bedding,
Table 2. Statistical analysis correlation between broiler’s intestinal alterations traits and summer season.

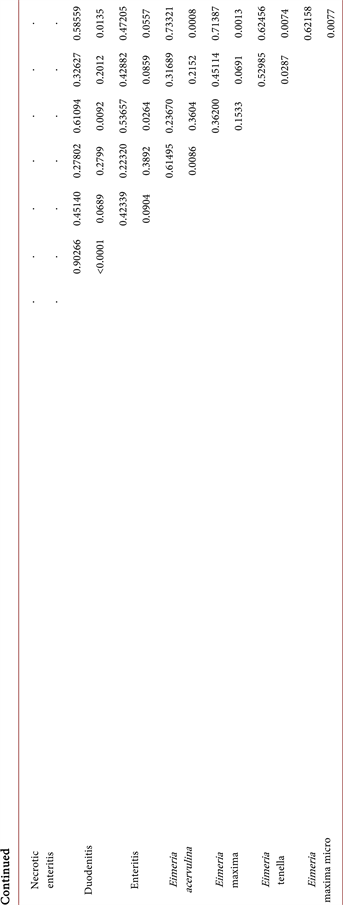
Table 3. Statistical analysis correlation between broiler’s intestinal alterations traits and autumn season.


Table 4. Statistical analysis correlation between broiler’s intestinal alterations traits and winter season.


Table 5. Statistical analysis correlation between broiler’s intestinal alterations traits and spring season.


passage of food, Turkish towel appearance, duodenitis, and enteritis (P = 0.05).
The presence of gizzard erosion was observed to be associated with the following signs throughout all four seasons: shedding of intestinal cells, excessive fluid and mucus, ingestion of bedding material, intestinal thinning, food passage, presence of Turkish towel appearance, duodenal inflammation, and the four Eimeria species. Nevertheless, there was a correlation between the consumption of mealworms and the erosion of the gizzard during the summer (P = 0.0426). Otherwise, during the spring and winter, there was a significant association between mealworm intake and thickening of the gut (P = 0.0060; P = 0.0018). In fall, there was a significant correlation with altered intestinal tone (P = 0.0003), and in autumn, winter, and spring, there was a correlation with enteritis (P = 0.05). The aforementioned results are summarized in Table 1. Additionally, E. maxima micro and E. acervulina exhibited higher prevalence rates (mean) during the winter (52.83% and 26.42% respectively). E. maxima also had a prevalence of 11.31% in the spring, while E. tenella had a prevalence of 6.74% in the autumn (Table 6). Hence, the presence of E. maxima microorganisms was observed to be higher during the winter season compared to the summer season (P = 0.0491). No significant variations were observed in the occurrence of other Eimeria species across different seasons (Table 6).
The results analysis revealed a correlation between Eimeria acervulina and Eimeria maxima during the summer, winter, and spring seasons. During the winter and spring seasons, a co-occurrence of Eimeria acervulina with Eimeria tenella was observed, while a co-occurrence of Eimeria acervulina with Eimeria maxima micro occurred during the same seasons. Eimeria maxima exhibits a correlation with Eimeria acervulina, as well as a correlation with Eimeria tenella in the autumn and spring, and with Eimeria maxima micro in the spring. Furthermore, E. tenella shows a correlation with E. maxima micro in the spring, in addition to the aforementioned correlations.
4. Discussion
The occurrence of necrotic enteritis in the spring was positively associated with an abundance of fluid in the last portion of the intestine. However, there was no significant link between specific necrotic enteritis and the presence of E. acervulina, E. tenella, E. maxima, and E. maxima micro. Santiani et al. [10] observed
![]()
Table 6. Seasonal variation in the prevalence of Eimeria infection (%).
Means followed by different letters on the lines differ by Tukey’s test (5%).
that 93.8% of the studied batches with subclinical necrotic enteritis signs tested positive for coccidiosis. The macroscopic lesions of coccidiosis were classified as grade 1 for E. acervulina (27%), E. tenella (9.7%), and E. maxima (8.9%). The histopathological examination revealed no necrotic tissue, but instead showed bleeding in the mucosa and submucosa, along with the presence of Eimeria spp. The absence of Clostridium perfringens type A netB+ indicates that the macroscopic lesions primarily observed in the jejunum were not indicative of necrotic enteritis, as confirmed by histology and the absence of the NetB gene. This finding aligns with our correlation analysis, which indicates that necrotic enteritis is not correlated with E. acervulina, E. tenella, E. maxima, and E. maxima micro. There was a consistent relationship observed between gizzard erosion and intestinal alterations in broiler chickens over the majority of seasons throughout the year. Roppa et al. [11] reported that Aviadenovirus, particularly type 1, is present in 75% of asymptomatic birds, and this is associated with the occurrence of Gizzard Erosion Syndrome (GES). It is worth mentioning that, apart from gizzard erosion, additional alterations occur in the gastrointestinal system of birds across the seasons. The intake of mealworms has been associated with the occurrence of fine intestine, E. maximum micro, and gizzard erosion throughout the summer. In autumn, the presence of worms was correlated with gizzard erosion, and in spring, the presence of Turkish towel appearance was associated with all these conditions. The mealworm undergoes its reproductive cycle, from incubation to adulthood, throughout a span of 55 days at a temperature of 27˚C [12] . Cold temperatures (below 16.5˚C) might effectively help control these insects by preventing the development of their immature stages, resulting in a drop in population [13] . During the warmer seasons, there is an increased abundance of insects, leading to a higher likelihood of birds consuming them and therefore experiencing gizzard erosion. Goodwin and Waltman [14] collected darkness beetles (Aplhitobius diaperinus) from seven premises (farms). Almost all picked Aplhitobius diaperinus were shown to contain myriad infectious organisms including dangerous bacteria like Salmonella spp., viruses (e.g., reovirus), and Eimeria (the causative agents of intestinal coccidiosis). The correlation observed between E. maxima micro and summer season could be explained by the phenomenon aforementioned by Goodwin and Waltman [14] .
During different seasons, E. acervulina was most prevalent in winter, E. maxima in spring, and E. tenella in fall. The prevalence of E. acervulina, E. maxima, and E. tenella during the course of the year was found to be 13.45% in winter, 13.27% in spring, 11.88% in autumn, and 8.16% in summer. According to the findings of Ahad et al. [5] , coccidiosis is most prevalent during the fall season, with a prevalence rate of 45.12%. It is less prevalent during the summer (30.84%), spring (23.81%), and winter (20.29%) seasons. Razmi and Kalideri [4] found that the occurrence of subclinical coccidiosis in broiler chickens in Iran was more common throughout the spring and winter seasons than during the fall or summer seasons. Based on our research conducted in Brazil, the prevalence of subclinical coccidiosis was highest during winter (52.83%), followed by spring (49.5%), autumn (41.63%), and summer (25.88%). In their study, Awais and Akhtar [15] found that the condition had the highest prevalence during the fall season (60.02%), followed by summer (47.42%), spring (36.92%), and winter (29.89%). Seasonal variation in the prevalence of Eimeria infection may be attributed primarily to the diverse effects of each season in the evaluated geographical locations, rather than to intrinsic factors of the disease itself. Differences in environmental humidity are likely a key factor contributing to the variations in incidences observed. The high occurrence of rainfall during the winter, spring, and autumn seasons may contribute to the higher incidence observed. Increased humidity facilitates the proliferation of oocysts by creating a more favorable environment for their sporulation and survival in the litter [4] . The occurrence of this disease throughout the year is influenced by both relative humidity and environmental temperature, which directly affects the oocyst sporulation rate [15] . Bachaya et al. [16] found that the occurrence of coccidiosis decreases when the surrounding humidity diminishes. Due to the increased relative humidity during the rainfall months, it has been found that the maximum occurrence of this phenomenon takes place in Pakistan in September, October, December, and January.
In a study conducted by Schneiders et al. [17] , it was discovered that heat stress led to a decrease in the release of E. maxima oocysts in birds. Schneiders et al. [17] found that this decrease is associated with reduced expression of genes involved in gamete fusion and replication. The decreased prevalence reported in our summer survey (25.88%) can be attributed to this feature.
According to Taylor et al. [18] , the most reliable indicators of Eimeria infection are the intestinal lesions. Avian coccidiosis is recognized for its ability to reduce body weight gain, deteriorate feed conversion, and increase crypt depth within the small intestine [19] . During the whole life cycle of the parasite, each oocyst has the potential to harm 2048 enterocytes [9] . Galli et al. [2] have been shown that coccidiosis disrupts the intestinal energy balance by reducing ATP levels, attributed to an increase in the production of reactive oxygen species (ROS). Coccidiosis enhances intestinal permeability, upregulates claudin junction protein expression, and concurrently decreases villus height by 20% [20] . These alterations facilitate the entry of viruses and toxins into the intestinal lumen, impeding nutrient absorption and stimulating intestinal mucus production. Consequently, modifications occur in the gastrointestinal system of birds, resulting in a decrease in food absorption. Indeed, coccidiosis induces an increase in the rate of intestinal protein metabolism, leading to significant energy expenditure as a consequence of inflammation. Previously, Gazoni et al. [9] established a correlation between E. maxima and E. acervulina and the occurrence of gizzard erosion; potentially due to broiler chickens ingesting a greater amount of bedding material. Gastrointestinal diseases commonly lead to an elevated rate of nutrient transit. This phenomenon arises due to inflammation in the intestines, resulting in excessive mucus production. As a result, enterocytes struggle to absorb nutrients effectively. Therefore, it is crucial to emphasize the significance of examining intestinal abnormalities in the field and understanding their association with coccidiosis and other diseases. The insights gained from this study would be valuable in developing customized control strategies and preventive programs for managing coccidiosis in poultry, particularly in regions with similar climates and poultry production systems.
5. Conclusion
Seasonal variations in coccidiosis incidence showed a clear correlation, with statistically significant data observed, typically at least P = 0.05. From 2012 to 2018, E. maxima micro and E. acervulina were prevalent during the winter, E. maxima during the spring, and E. tenella during the autumn. Furthermore, regardless of the season, Eimeria maxima micro was found to be more prevalent in the Brazilian poultry industry compared to other Eimeria species. The presence of Eimeria species led to lesions that affected various factors related to the health of the bird’s intestines, including shedding of intestinal tissue, erosion of the gizzard, digestive system transit rate, and excessive mucus production, among other effects. Therefore, these statistics are valuable for experts in the poultry industry to develop different strategies for managing this disease based on seasonal variations, thus minimizing financial losses.
Funding
This research was supported in part by funds provided by USDA-NIFA, Sustainable Agriculture Systems, grant no. 2019-69012-29905. Title of project: Empowering U.S. Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): no. 2019-69012-29905.