Antibiotics Resistance of Urogenital Mycoplasma in Sexually Active Women Attending Gynecologic Consultation in Douala (Cameroon) ()
1. Introduction
Urogenital mycoplasmas are bacteria that are commonly found in the commensal flora of humans and can occasionally be harmful [1] . These microorganisms are frequently linked to a number of diseases, including reproductive disorders, cervicitis, non-gonococcal urethritis, premature rupture of membranes during pregnancy, neonatal infections and bacterial vaginosis [2] [3] . Currently, urogenital infections have been linked to at least five different species of mycoplasma; the most common species are Mycoplasma hominis and Ureaplasma urealyticum, while the less common are M. genitalium, M. fermentans and M. penetrans [4] . Mycoplasma infections epidemiological data continue to differ depending on the study population and geographical area; for instance, genital swabs from men in China showed a prevalence of 43.7% genital mycoplasma, and 24.6% was recorded in Padua, Italy [5] [6] . Mycoplasma infection rates are still high in Sub-Saharan Africa [7] . The combined frequency of Mycoplasma hominis and Ureaplasma urealyticum in vaginal swabs was 7% in a 2009 study conducted in Burkina Faso [7] . In Benin, 35.1% of people tested positive for mycoplasma [8] . It was discovered that 71.4% of women living with Human Immunodeficiency Virus (HIV) in Cameroon also had mycoplasma infections [9] . Two comparable studies, one in Douala among sexually active women and the other one in Yaoundé among pregnant women, reported a prevalence of 65% and 38% respectively [10] [11] . Despite the existing therapeutic arsenal that includes antibiotics from the Cyclin, Macrolide-lincosamide and Fluoroquinolone families, it has been noted that Mycoplasma is developing acquired resistance to these widely used antibiotics [12] [13] . While Clarithromycin, Josamycin and Doxycycline remained active, a notable rate of resistance to Ciprofloxacin, Ofloxacin, Azithromycin and Erythromycin was observed in Italy and Switzerland [14] [15] . Josamycin showed good activity against all the species in Côte d’Ivoire, including M. hominis and U. urealyticum, whereas Fluoroquinolones showed poor activity [2] . The degree of resistance differs from one country to another, based on treatment guidelines and even between populations [2] [13] . In genital swabs from HIV-positive women, the susceptibility to Fluoroquinolones declines with time (20% for ofloxacin and 18.3% for ciprofloxacin); the majority of Mycoplasma isolates are resistant to Erythromycin, Roxithromycin, Clindamycin, Ciprofloxacin and Clarithromycin in pregnant women [9] [10] .
The aim of this study was to determine the degree of antibiotic-resistant Mycoplasma in genital swabs from sexually active women attending the Douala General Hospital.
2. Methodology
2.1. Type of Study, Location and Study Period
From January to June 2022, a cross-sectional study was carried out at the Douala General Hospital (DGH). Samples were collected in the Department of Gynecology and examined in the bacteriology unit of the Clinical Biology Laboratory.
2.2. Study Population
These were sexually active women that visited the DGH for consultation in the Department of Gynecology and those seen at the Douala General Hospital laboratory.
After obtaining their informed consent, women who agreed to participate and who had not received any antibiotic therapy (aminoglycosides, macrolides, quinolones) for a period of at least one week were selected. Women who were menstruating, those who had had sex for less than 24 hours and/or who had taken a vaginal shower the morning of the sampling were not included. The Cochrane formula was used to determine the minimum participant size.
2.3. Data Collection
Data were collected using a pre-tested individual survey form, including socio-demographics such as age, marital status and socio-professional category. Physiological criteria like pregnancy and menopause were assessed, as were clinical variables such as use of contraceptives, intimate gel and vaginal cleansing, pelvic pain, vaginal discharge and history of sexually transmitted infection.
2.4. Sample Collection
An endo-cervical genital specimen was taken from each woman using a sterile swab.
2.5. Seeding and Incubation of the Gallery
The colorimetric-based Mycoplasma System PlusTM (Liofilchem) gallery, for the detection, semi-quantitative counting, presumptive identification and antibiotic susceptibility test of Mycoplasma hominis and Ureaplasma urealyticum, was used according to the manufacturer’s recommendations. It also enabled the detection and presumptive identification of other microorganisms such as Trichomonas vaginalis and Candida spp.
After incubation at 36˚C ± 1˚C for 24 hours (and up to 48 hours in case of negativity on the first day) in an oven, Ureaplasma spp. counts were indicated by a color change from yellow to red in the corresponding wells (103, 104 and ≥105), as was Mycoplasma hominis (104 and ≥105).
The presence of Trichomonas vaginalis and Candida spp. was determined microscopically (40×) by examining culture fluid taken from the corresponding wells (presence of flagellated motile trophozoites for T. vaginalis and/or the presence of chlamydospores and hyphae for Candida spp).
Antibiotic susceptibility testing of urogenital mycoplasmas, based on 9 antibiotics (Tetracycline, Pefloxacin, Ofloxacin, Doxycycline, Erythromycin, Clarithromycin, Minocycline, Clindamycin, Azithromycin) was assessed by observing a color change from yellow to red in the corresponding wells.
2.6. Statistical Analysis
The data were analyzed using Epi Info software version 7.2.2.1. The Chi2 test was used for comparison of categorical variables and the Student T-test for continuous quantitative variables (mean, median). Values of p-value < 0.05 were considered significant and the strength of association was delivered as an odds ratio with 95% CI.
3. Results
A total of 187 women were targeted in the gynecology and laboratory departments and 107 were recruited according to inclusion criteria.
Age
The median age of the study population was 33 ± 8.3 years with a minimum of 21 years and a maximum of 59 years. The most represented age group was 30-40 years, i.e. 41.1%.
Socio-professional category
The majority of the study population (63.6%) was salaried. Students represented 22.4% of the participants.
Marital status
According to marital status, the most represented class was married women (52.3%).
Region of origin
An analysis by region of origin shows that in this sample of patients, the most represented region was the West (35.5%).
Number of sexual partners
Of the 107 women we interviewed, 85% had a single sexual partner, 11.2% had two, 1.9% had 3, and 1.9% had 4 partners.
Physiological condition
Analysis of the women’s condition showed that 18.7% were pregnant and 6.5% were already menopausal.
Clinical and Para clinical features
Reason for consultation
Some women presented several reasons for consultation. The most common reason was routine or premarital check-ups (29.9%) and the prenatal consultation of pregnant women represented 17.8%.
History of sexually transmitted infections (STIs)
The results showed that 39.3% of women had already contracted an STI such as chlamydia, herpes or mycoplasma.
The percentage of women using no form of contraception method was higher (82.2%), compared to those using intrauterine devices (8.4%), condoms (6.5%), orals (2.5) and then injectable (0.9%) (Table 1).
Frequency of mycoplasma infection
The proportion of women with urogenital mycoplasma infection was 73.8%.
Candida albicans was found in 31.7% of cases, none of the women tested were carriers of Trichomonas vaginalis and 15.9% of the study population had no infections (mycoplasma, Candida spp, Trichomonas vaginalis).
The age group most infected with Ureaplasma urealyticum and co-infection was 30 to 40, with 37.5% and 56.3% respectively.
Among women who tested positive for urogenital mycoplasma infections, those with co-infection (Ureaplasma urealyticum and Mycoplasma hominis) were in the majority, accounting for 54.4% of cases (Table 2).
Resistance profile of urogenital mycoplasma to the antibiotics tested
Minocycline was the most active antibiotic for Mycoplasma hominis (resistance rate 0%). There was a high level of resistance to Erythromycin, Clarithromycin, Azithromycin and Tetracycline - 100% for the first two, 88.8% and 77.8% respectively.
Clarithromycin was the most active antibiotic for Ureaplasma urealyticum (resistance rate 3.7%). A high rate of resistance to clindamycin (100%), followed by tetracycline (33.3%), was observed for this same species.
Based on the results of the antibiotic resistance profile, Minocycline was the most active antibiotic for cases of co-infection (resistance rate 9.3%).
The rate of resistance to erythromycin was 100% in cases of co-infection and Mycoplasma hominis infection; 100% and 97.7% for clindamycin in Ureaplasma urealyticum infection and co-infections respectively (Figure 1).
The most active antibiotic for all colonizing species was Minocycline.
Factors associated with mycoplasma infection
There was no significant association between factors such as age, socio-professional categories, marital status, hormonal status, non-use of contraceptives, number of sexual partners and Mycoplasma infection (Table 1).
Factors associated with Clarithromycin antibiotic resistance
In univariate analysis, four parameters were independently associated with Clarithromycin resistance; such as Pelvic pain, Use of intimate gel resistance, inflamed cervix and vaginal cleansing. However, in multivariate analysis, only Pelvic pain can be associated with this resistance with OR = 3.054 [1.311 - 7.1186], p = 0.010 (Table 3).
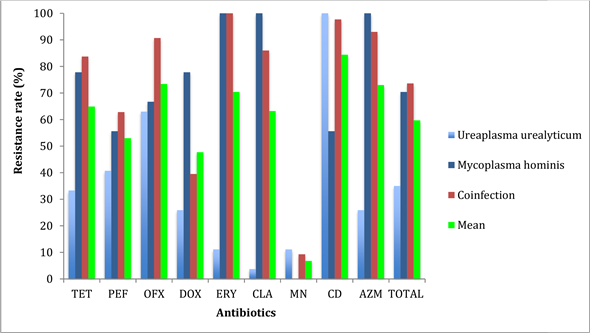
Resistance of the various strains to the antibiotics tested. TET, Tetracycline; PEF, Cefepime; OFX, Ofloxacin; DOX, Doxicycline; ERY, Erythromycin; CLA, Clarithomycin; MN, Minocycline; CD, Clindamycin; AZM, Azitromycin.
Figure 1. Antibiotic resistance profile of different strains (N = 79).
![]()
Table 1. Factors associated with Mycoplasma infection.
IUD = Intra Uterine Device.
![]()
Table 2. Frequency of pathogens in cultures and microscopy.
![]()
Table 3. Factors associated with resistance to clarithromycin.
4. Discussion
The study population consisted of 107 women aged between 21 and 59 years. The age group with the highest rate of Ureaplasma urealyticum infection and co-infection was the 20 - 30 age group, with 56% and 56.4% respectively. There is a similarity with the results of work carried out in Mali by Guindo et al. in 2022, who showed that the most infected patients belonged to the 16 to 35 age group (45.2%) [16] .
Half of the study population was represented by married women (50%), followed by single women (31%), the majority of whom were salaried employees (63%). These results differ from those of Tebeu et al. found in a study carried out at the Yaoundé Gyneco-Obstetric and Pediatric Hospital in 2020, where the majority of women were students (24.8%) [17] .
Microbiological analyses showed that, in terms of physiological status, the frequency of mycoplasma infections was highest in post-menopausal women (85.7%), followed by non-pregnant women (75%).
The prevalence of mycoplasma infection was 73.8%, higher than those obtained by Longdoh et al. in 2011 in Yaoundé, Cameroon, Ndjamena, Chad, and Leli et al. in Italy (65%, 27% and 24.6% respectively) [10] [5] [18] . This difference can be explained by the fact that mycoplasma infections remain variable depending on the study population and geographical area [5] .
The most infected age group corresponds to that in which women are more sexually active.
The rates of colonization by Ureaplasma urealyticum, Mycoplasma hominis and co-infection in the study population were 25%, 9% and 39% respectively. In contrast, Bolti et al. found in 2022 that U. urealyticum was predominant, with an identification rate of 74% versus 21.7% for M. hominis; similarly, U. urealyticum/M. hominis co-infection was superior to M. hominis mono-infection [18] .
Trichomonas vaginalis, which we did not find in our series, could also be detected. The T. vaginalis/Mycoplasma grouping is an example of a combination of microorganisms of low virulence, which, together, are capable of producing potentially aggressive diseases [19] [20] . The symbiosis between T. vaginalis and M. hominis represents the first reported and unique case of association between two obligate parasitic organisms of man, capable of producing infections in the same anatomical site and causing independent diseases [19] . Mycoplasma hominis can influence the pathogenicity of T. vaginalis, suggesting a role for symbiosis in the variability of signs and symptoms observed during trichomoniasis [20] .
Ureaplasma urealyticum strains were highly sensitive to macrolides: 88%, 96% and 72% respectively for erythromycin, clarithromycin and azithromycin. In contrast, strains of Mycoplasma hominis were multi-resistant to the same family of antibiotics (i.e. 0% sensitivity to each). Co-infections also showed low levels of sensitivity to the macrolides tested. Ureaplasma urealyticum was totally resistant to Clindamycin. However, there is a discrepancy with the study carried out in 2019 by Kouassi et al. who found that 81.5%, 33.3% and 22.2% of M. hominis strains were sensitive to azithromycin, clarithromycin and erythromycin respectively [2] .
Minocycline was the most active molecule whatever the germ considered, and even in co-infection, with sensitivities of 92%, 100% and 92.3% respectively for U. urealyticum, M. hominis and co-infection. The other 2 cyclins remained highly effective against U.urealyticum, with 68% of strains sensitive to tetracycline and 80% to doxycycline. However, activity against Mycoplasma hominis and the 2 co-infected germs was lower (22.2% and 22.2%) and (17.9% and 66.7%) respectively. These results are similar to those of Zhu et al. in 2016 in China, where cyclins were the molecules that showed the best activity on all mycoplasmas compared with other antibiotic families [6] .
The level of cyclin resistance was highest with M. hominis, at 77.8%, 33.3% and 0% respectively for tetracycline, doxycycline and minocycline; for U. urealyticum, it was 32% for tetracycline, 16% for doxycycline and 4% for minocycline. This level of resistance is higher than those obtained in Dakar by Diop-N’diaye et al. in 2014 with rates that varied between 5% and 14% [21] . Our results also diverge from those obtained in Burkina Faso by Karou et al. and in Cuba by Diaz et al. with tetracycline and doxycycline resistance rates of 20% and 25% respectively for U. urealyticum and 40% and 35% for M. hominis [7] [22] . The Fluoroquinolone family was the least resistant, although these rates remain high. However, these differences could be explained by the recurrent practice of self-medication in our countries and by the different infection management policies in place [23] .
Age, socio-professional and marital status, physiological state, non-use of contraceptives and number of sexual partners, were factors associated with mycoplasma infection, but were not significant. Similarly, Zhang et al. showed in their study that there was no association between mycoplasma infection and marital status, occupation, smoking, alcoholism, age, pregnancy and menopausal status [24] .
Vaginal cleansing is a factor significantly associated with the resistance of urogenital mycoplasmas to antibiotics. This could be explained by the fact that vaginal cleansing may be responsible for an imbalance in vaginal flora, thus promoting mycoplasma colonization of the genital tract [25] .
5. Conclusion
The study showed that, of the species isolated, Ureaplasma urealyticum had good sensitivity to most macrolides, cyclins and quinolones Mycoplasma hominis and co-infections were multi-resistant to all these antibiotics except minocycline. It is therefore important to study the sensitivity of each mycoplasma species to antibiotics in cases of co-infection. Vaginal douche is a risk factor for vaginal infection.
Study Limits
The size of the sample and the fact that this phenomenon was studied in a single structure mean that the conclusions of this work cannot be generalized.
Acknowledgements
We would like to express our gratitude to all the women who agreed to take part in this study.
Author Contributions
JPNM, COE and CTN coordinated the study; JPNM, RFN, FMN and COE drafted the manuscript; RFN, ERM and GDN collected the data, performed the laboratory analyses and interpreted the results. JPNM and TNJ participated in the study design. COE, ENM and ERM carried out the statistical analysis. All authors read and approved the final manuscript.
Ethics
The study was conducted in accordance with the ethical guidelines for research in Cameroon. We obtained research authorizations from the Director of Douala General Hospital, and ethical approval from the Institutional Human Health Research Ethics Committee of the University of Douala (N˚ 3010 CEI-UDo/04/ 2022/M).