Concomitant Acute Coronary Syndrome and Acute Ischemic Stroke: A Particular Association and a Therapeutic Dilemma ()

1. Introduction
Acute ischaemic stroke (AIS) and acute myocardial infarction (AMI) are life-threatening diseases that can lead to permanent morbidity or disability [1]. They share common vascular risk factors that are risk factors for atherosclerotic disease. The occurrence of one can be a complication of the other and vice versa. One in five patients with an ischaemic stroke will experience a serious cardiac event within weeks [2]. Most studies have determined an increased incidence of ischaemic stroke after a previous myocardial infarction, both in the short and long term. This has been attributed in the vast majority of cases to embolisation due to left ventricular wall thrombi or atrial fibrillation [3]. Cardiocerebral infarction (CCI), a term introduced by Omar et al. in 2010 [3], has been used to describe the simultaneous occurrence of AIS and AMI. This type of infarction is uncommon and represents a real therapeutic dilemma for both cardiologists and neurologists, as well as an increased risk of mortality for the patient. Both conditions have a narrow therapeutic window, so that acute management of one at the expense of the other may result in irreversible permanent disability of the infarcted area due to late intervention. In addition to the fact that the use of antiplatelet and anticoagulant agents in the management of AMI prior to percutaneous coronary intervention (PCI) may increase the risk of haemorrhagic conversion associated with intravenous thrombolysis [4], the use of a thrombolytic in an AIA increases the risk of cardiac wall rupture in AMI [5].
2. Cases
2.1. Case Presentation 1
This is a 46-year-old patient admitted to the emergency department for typical chest pain evolving for 48 hours without any other symptoms. She has been hypertensive for 8 years on Amlodipine 5 mg, type 2 diabetic for 10 years poorly balanced on oral antidiabetic, sedentary with abdominal obesity. In his antecedents, there was a notion of cerebral vascular accident 6 years earlier with total recovery without sequelae. The examination noted a hemodynamically and respiratory stable patient with a blood pressure of 140/80 mmHg, a pulse of 75 bpm and a respiratory rate of 17 breaths/minute. Cardiovascular examination revealed a murmur in the left carotid artery. The rest of examination was normal.
The electrocardiogram showed a sinus rhythm of 64 beats/minute, and an elevation of the ST segment of the apical and lateral leads with negative T wave in the same leads (Image 1).
Transthoracic echocardiography showed a hypertrophic left ventricle with preserved systolic function (LVEF = 60%) without segmental hypokinesia without thrombus. Laboratory investigations revealed a serial elevation of cardiac enzymes to 594 μg/L (50 times normal). The rest of the biological assessment was normal. Coronary angiography was performed showing thrombotic occlusion of the proximal segment of the circumflex coronary artery, severe stenosis of the middle segment of the left anterior descending artery, a severe stenosis of the distal segment of the right coronary artery (Image 2). The management consisted of angioplasty of the proximal segment of the circumflex artery coronary artery with indication of bypass on the other lesions in a second step, all under cover of antiplatelet and anticoagulant treatment.
Within 24 hours of admission, she presented with neurological signs with right hemiplegia, global aphasia, and an NIHSS score of 9. Immediate magnetic resonance imaging was consistent with stroke left anterior choroidal ischemia (Image 3). The thrombolysis performed successfully at H1 of the symptomatology by Tenecteplase according to his weight with total recovery and the NIHSS score went from 9 to 0 after 1 h 30. Ultrasound of the supra-aortic trunk revealed a tight stenosis of more than 70% in the left common carotid bulb with a
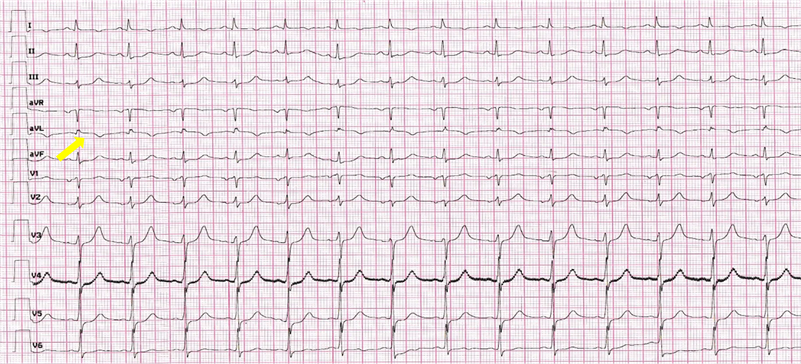
Image 1. Electrocardiogramm showed slight ST elevation in apical and lateral leads with T wave inversion (yellow arrow).
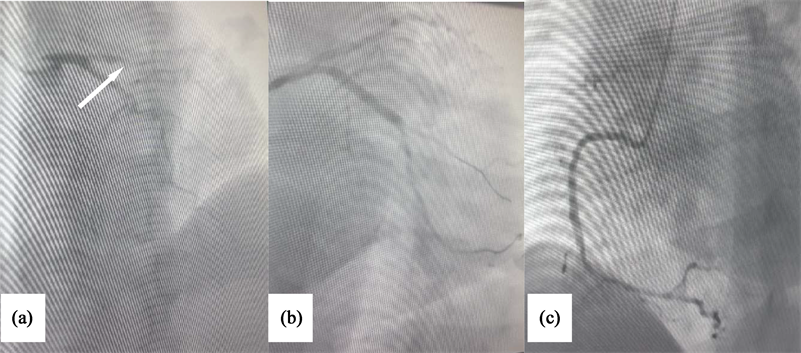
Image 2. Emergency coronary angiogram shows thrombotic occlusion of the proximal segment of the circumflex coronary artery (a), tight stenosis of the medium segment of anterior interventricular artery (b), tight stenosis of the distal segment of right coronary artery (c), after percutaneous coronary intervention.

Image 3. (a) Cerebral scanner show large ischemic territory (yellow arrow); (b) and (c) Magnetic resonance imaging showed a left anterior choroidal stroke (yellow arrow).
good downstream network requiring endarterectomy.
2.2. Case Presentation 2
A 62-year-old patient presented to the emergency department for angina pain for 24 hours, aggravated 4 hours before admission, becoming infarctoid with the concomitant occurrence of dysarthria, slight hemiparesis with central facial paralysis. In his history, there was essentially hypertension for 3 months and type 2 diabetes for 10 years. The clinical examination revealed grade 3 arterial hypertension of 170/95 mmHg, symmetrical and a regular pulse at 110 per minute with hyperglycemia at 4.1 g/dl. Cardiovascular examination revealed signs of killip 2 left ventricular failure suppressed by injectable Furosemide. The electrocardiogram showed a sinus rhythm of 110 beats/minute, a QS pattern, and ST-segment elevation in the inferior and basal leads extended to the right leads with an anteroseptal and lateral mirror image (Image 4).
Transthoracic echocardiography showed a left ventricle with moderate dysfunction at 44% with inferior and inferoseptal hypokinesia on the basal and middle segment, inferior and septal hypokinesia on the apical wall; high filling pressures due to heart failure. The cerebral scanner (Image 5) objectified an AIS of left insular in the left middle cerebral artery territory with an NIHSS score of 3. The patient was out of thrombolysis time for AIS and AMI. Coronary angiography showed total occlusion of the proximal right coronary artery, tight stenosis of the distal main trunk with tight stenosis of the three segments of the anterior descending artery. She was treated by angioplasty of the right coronary artery with indication of revascularization of the left network in a second step (Image 6). Control imaging within 5 days showed the persistence of hypodensity in the left middle cerebral artery without hemorrhagic transformation. The rest of the explorations could not be done because of the evolution was fatal.
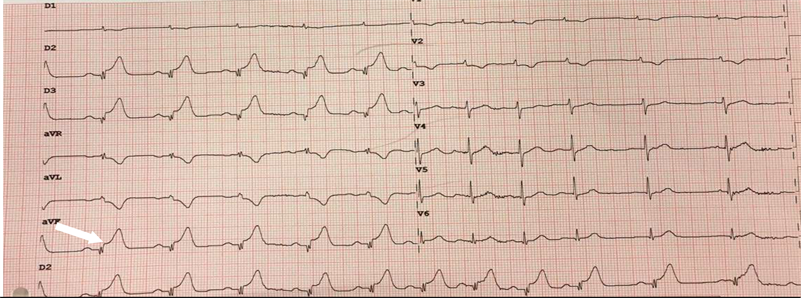
Image 4. Electrocardiography showed a QS complex and ST segment elevation in inferior (white arrow) and with mirror image in anteroseptal and lateral leads.
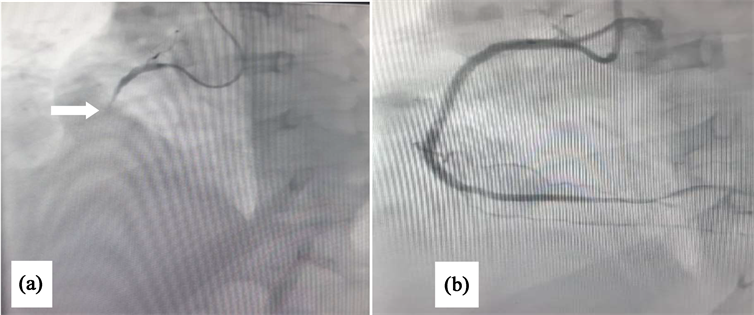
Image 5. Emergency coronary angiogram shows: (a) total occlusion of the proximal right coronary artery before stenting (white arrow); (b) after percutaneous coronary intervention; (c).
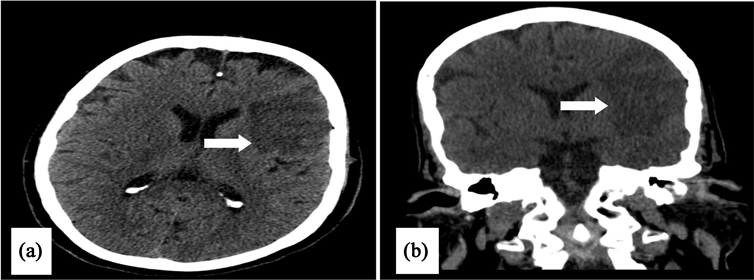
Image 6. Computed tomography angiogram (CTA) objectified an ischemic stroke of the middle left cerebral artery (a) and (b).
3. Discussion
3.1. Epidemiology
The reported prevalence and incidence have mostly been for the metachronous presentation of CCI. The incidence of a simultaneous CCI is currently unknown due to the rarity of this co-occurrence [6]. In a prospective study published in 1984, Rokey et al. [7] reported the prevalence of CAD as 58% among patients presenting with transient ischemic attack and AIS compared with 7% in other age matched patients in the same institution. Chin et al. [8] reported the incidence of CCI as 12.7% in geriatric patients who were screened for AMI within 72 hours of admission for acute stroke. Findings from the Global Registry of Acute Coronary Event (GRACE) trial reported an incidence of in-hospital stroke as 0.9% in a cohort of patients presenting with acute coronary syndrome, and the incidence was much higher in patients with STEMI than the non-STEMI [9]. The most recent data in 2017 from Yeo et al. showed that 6% of patients with acute stroke had ST segment elevation [10].
3.2. Pathogenesis of CCI
The mechanism of this life-threatening situation is unclear and may be multifactorial [11]. There are four major pathophysiologic mechanisms of CCI including 1) cardioembolic, 2) hypotensive 3), neurogenic stunned myocardium, and 4) dissection.
AMI, especially anterior and apical wall infarction associated with reduced left ventricular systolic function and the presence of a severely hypokinetic left ventricular myocardium segment also increases the risk of thrombus formation [6] [12] which may embolize simultaneously to both coronary and cerebral arteries.also, the risk is not negligible in patients suffering from atrial fibrillation [13], and likewise is the possibility of a paradoxical embolus of a right ventricular thrombus or a deep vein thrombosis through a patent foramen ovale [14].
Hypotension, which occurs as a sequelae of AMI in patients presenting with AMI and long-standing history of hypertension may result in reduction of cerebral blood flow to water-shed areas of the brain and subsequent infarction, especially if there is a failure of blood pressure auto-regulatory mechanisms [3]. In a study evaluating the relationship between low-normal systolic blood pressure levels (<120 mmHg) and the risk of recurrent stroke in patients with recent non cardio-embolic ischemic stroke, there was increased risk of recurrent stroke in patients with low-normal systolic blood pressure compared to normotensive patients [15].
Other potential mechanism of a CCI is an AIS involving the left insular cortex. Left insular damage is thought to impair sympatho-vagal balance resulting in cardiac arrhythmias and wall motion abnormalities [6]. Also, adrenergic surge associated with AIS may result in catecholamine induced myocardial stunning, a common cause of stress induced cardiomyopathy (Takotsubo syndrome) that may mimic ST elevation AMI, and in turn favors formation of intra-cardiac thrombus that may embolize to the cerebral and coronary arteries [16].
Although rare, extension of an ascending aortic dissection to the coronary ostia and a subsequent extension to the carotid, vertebral, or basilar arteries may also explain the simultaneous occurrence of a cerebral and a coronary infarction [17].
Moreover, there have been several case reports of CCI attacks in special circumstances, such as aortic dissection, electrical injury, marijuana abuse, or underlying acute myeloid leukemia [18]. In some cases, a CCI attack may also occur due to atherosclerosis in the cerebral and coronary arteries, respectively
3.3. Diagnostic and Management
The best approach to diagnose and treat acute coronary syndrome (ACS) in acute ischemic stroke (AIS) patients is unknown. Presentation of signs and symptoms may differ in AIS patients due to impaired cognition, communication, sensation and perception. ACS may even be clinically silent in AIS patients. Guidelines recommend measuring troponin in AIS patients, but diagnostic and therapeutic consequences remain vague [19].
There are no evidence-based guidelines or clinical studies on the management of the co-occurrence of AIS and AMI, especially in the priority of the treatment [20]. The agents of management for each territory may complicate the extent of the other infarcted territory. Antiplatelet therapy [21], GPIIa/IIIb inhibitors [21] and anticoagulants [22] used in coronary intervention for AMI increase the risk for hemorrhagic conversion of AIS associated with thrombolytic, and the use of a thrombolytic in AIS increases the risk of cardiac wall rupture in setting of AMI [5]. The ideal management of simultaneous CCI is a treatment strategy that benefits both vascular territories. Intravenous thrombolysis, approved for the acute management of both conditions has been suggested as the best approach to the treatment of simultaneous CCI if there is no contraindication, and both presentations are within the time frame for the administration of a thrombolytic [6] although this has not been studied in clinical trials nor supported by any societal guidelines. The challenge to this management approach is the different dosage and duration of thrombolytic administration recommended for treatment of acute infarction of these vascular territories.
Guidelines for the early management of patients with acute ischemic stroke, updated in 2019, recommend intravenous Alteplase at the dose used for cerebral ischemia followed by PCI for hyperacute co-occurrence of AIS and AMI (class IIa; level of evidence C) [23].
A combined endovascular approach with the use of percutaneous transluminal coronary angioplasty (PTCA) for AMI and thrombectomy devices for AIS have been suggested [10]. PCI is preferred over thrombolysis for AMI [24]. However, the use of adjunctive antiplatelet therapy with PCI poses a significant risk of bleeding with endovascular treatment for AIS.
Therefore, Management of CCI is individualized. Elochukwu et al. [17] summarized the cases reported in the literature and their optimal management, among the twenty-five cases reviewed within the literature review performed, 7 (28%) utilized mechanical thrombectomy (MT), 15 (60%) underwent Percutaneous intervention (PCI), 9 (36%) received fibrinolysis (Alteplase), and 12 (48%) had concurrent cardiac thrombus. 4 (16%) received mono antiplatelet (MAP) only, 3 (12%) DAP only, 4 (16%) MAP plus AC, 3 (12%) DAP plus anticoagulation (AC), 10 (40%) heparin therapy, and 8 (32%) AC other than, and/or with heparin. Outcomes were reported in 16 (64%), with 4 (16%) and 6 (24%) having Modified Rankin Score (MRS) ≤ 3 at 1 and 3 months respectively.
Regarding CCI necessitating acute intervention, we propose combined treatment of myocardial and cerebral vascular territories with the administration of Alteplase (0.9 mg/kg) followed by PCI with stenting if indicated [10]. Concomitant need for mechanical cerebral thrombectomy can then be assessed using a cerebral angiogram.
4. Conclusion
The appropriate management of CCI represents a great challenge for practitioners. There are currently no clinical trials or consensus guidelines for the concomitant management of AMI and AIS. It is necessary to identify a federative dose of intravenous thrombolytic, the optimal duration of administration, the role of antiplatelet agents and combined coronary and cerebral endovascular interventions.