Differential Effects of Cold and Heat Shock on Embryogenic Induction and Green Plant Regeneration from Wheat (Triticum aestivum L.) Microspores ()
1. Introduction
Among a number of published protocols for wheat anther/microspore cultures, nearly all employ some type of stress treatment to switch microspores from their naturally destined pollen formation to an alternative development pathway leading to in-vitro embryogenesis. The stress treatment may vary, including cold or heat shock, sugar starvation and/or inducer chemicals. Types of explants being treated may also vary, including live plants, excised tillers, spikes, florets, anthers and isolated microspores [1] - [6] . Cold shock has long been recognized as beneficial to anther cultures in cereal crops [1] [7] . The general strategy for cold pretreatment involves storing collected tillers at a low temperature (~4˚C) for several days prior to excising anthers for in-vitro cultures. Both higher callus yield and increased frequency in double haploids among regenerants were observed in anther cultures following a cold pretreatment [8] [9] [10] . Barley tillers were placed in a 4˚C refrigerator for as long as four weeks prior to anther/microspore cultures to improve double haploid production [11] .
Heat shock, alone or in combination with starvation, seemed to trigger or enhance microspore embryogenesis [3] [12] [13] [14] . When excised wheat anthers were subjected to high temperature (32˚C - 34˚C) pretreatment, higher callus yields were observed [15] [16] . The stress from elevated temperatures, coupled with nutrient starvation, resulted in an increase in embryoid yields from isolated tobacco microspores [3] . When excised wheat anthers were treated in a 33˚C incubator, higher yields of embryoids were also obtained from subsequent microspore culture, although plant regeneration (PR) and green plant (GP) frequencies were still low [13] . Later, a pretreatment that combined heat shock, nutrient starvation and inducer chemical(s) was found to further improve the efficiency of microspore cultures [14] [17] [18] . Starvation treatment of anthers in 0.3 M mannitol was also effective in inducing microspore embryogenesis [11] [19] .
Even with the most efficient system reported to date, lower green plant% or albinism in microspore cultures of cereal crops continue to hinder the wider use of double haploids in crop breeding. Among plantlets regenerated from microspore-derived embryoids, albino plants (AP) can range from 5% to 100%. Albinos among the regenerants from microspore cultures are common, especially among many genotypes and progenies that are of practical breeding values. High albinos are generally believed to be caused by either genetic or environmental factors or a combination of both, but the underlying mechanism is poorly understood. Genotype differences in GP/AP frequencies from anther cultures are well documented [20] [21] . The physical and chemical conditions during pretreatment and in-vitro culture are also implicated as contributing factors to low GP%. Some believed that low GP% might be caused by elevated incubation temperature in culture, which led to deletion of plastid genome [22] [23] . Others propose that the lack of some essential nutrients during stress treatment contributes to higher AP [24] .
Although GP% can be increased through genetic manipulation, the process is complex and time-consuming. Finding feasible means to improve GP% through altering environmental conditions during pretreatment and/or in-vitro cultures would be beneficial for the production and use of double haploids in crop breeding. Changes in physical/chemical conditions during pretreatment and/or in-vitro cultures can easily be implemented once optimal parameters are identified. Since an elevated temperature during pretreatment has been implicated as a cause for low GP%, and yet the heat shock at 33˚C is routinely used to induce microspore embryogenesis [25] , it would be valuable to find out if such heat shock is indeed responsible for low GP%. Another frequently employed pretreatment in anther/microspore culture is a cold shock for a longer period. Thus, the present study is designed to investigate the effects of temperature variations during both pretreatment and induction culture on microspore embryogenesis in general and on GP% in particular. The objectives for these experiments are three folds: 1) to test if various low-temperature pretreatments are effective in triggering microspore embryogenesis, as compared to the 33˚C heat shock; 2) to study if differential responses to cold vs heat pretreatment existed among genotypes, as measured by GP%; and 3) to find out if temperature variations during induction culture affect GP%. These results could be used to increase the GP% from microspore cultures.
2. Material and Methods
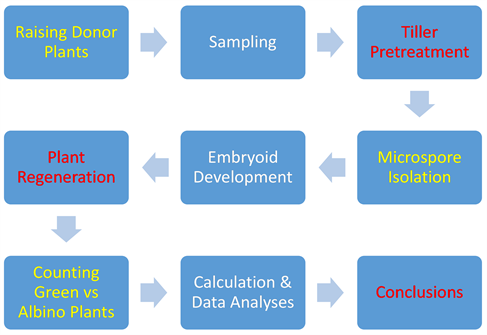
To understand this section better, a flow chart was created to illustrate the various stages of microspore cultures, from sample cultivation through green plant regeneration from embryoids.
2.1. Raising Donor Plants
Three genotypes were chosen for the study based on their varied GP% observed in earlier experiments [24] , with Calorwa yielding the lowest GP%, Pavon 76 next, and Chris the highest. Seeds of all three genotypes were obtained from USDA-ARS Small Grain Collection (Aberdeen, ID 83210). All plants were raised in an Enconair (Ottawa, Canada) GC-16 growth chamber. Details on raising microspore donor plants were described elsewhere [4] .
2.2. Collecting Tillers
Tillers were sampled when most microspores in anthers at the mid-section of a spike were at the mid- to late uninucleate stage [26] . Tiller sampling and processing were done according to the protocol by Zheng et al. [18] except for the temperature variations as described below.
2.3. Temperature Variations in Pretreatment
For cold shock pretreatment, flasks with standing tillers were incubated in refrigerators at four temperature levels: 4˚C, 7˚C, 10˚C and 13˚C for 2, 6, 10 and 14 days. Microspores were then isolated, collected and mixed with culture medium according to Liu et al. [14] . All petri dishes were incubated in an incubator at 28˚C for embryoid development. The numbers of embryogenic microspores were counted and estimated using a haemocytometer [26] . The number of embryoids for each treatment was counted at days 40, 45 and 50 after the initiation of each set of microspore cultures. Embryoids that were counted and transferred to the regeneration medium had reached a minimal of 0.2 mm in diameter. The smaller and yet-to-mature embryoids in each Petri dish were estimated and not transferred for plant regeneration. The numbers presented in Table 2 included both transferred and non-transferred embryoids. For heat shock pretreatment, flasks with standing tillers were incubated at 33˚C for 52 hours for both Calorwa and Pavon 76, and 72 hours for Chris, respectively. The two time-regimes at 33˚C temperature were deemed most effective for these three genotypes based on prior results [14] . Due to more rapid cell divisions and morphological differentiation by microspores from 33˚C pretreatment, it was necessary to count and transfer embryoids for plant regeneration at days 35, 40 and 45 after culture initiation.
2.4. Isolation and Culture of Microspores
Immediately following cold or heat shock pretreatment, tillers were disinfected in 20% commercial bleach and spikes were taken out of the boot. Protocols for microspore isolation, collection, induction culture and plant regeneration were the same as described in prior publications [14] [18] .
2.5. Temperature Variations during Embroid Development
For these experiments, only microspores isolated from 33˚C pretreatment were used. Isolated microspores were incubated at four temperature levels: 16˚C, 20˚C, 24˚C and 28˚C for embryoid development. Since microspores incubated at each of the four temperature levels are sub-samples from the same pool, embryogenic microspore% should be the same and thus said data was not collected. The numbers of embryoids were counted once they reach 0.2 mm in diameter and were then transferred to regeneration medium. The counting and transfers were done at 35, 40 and 45 days after culture initiation. Data on PR% and GP% were collected.
2.6. Data Analyses
All data presented were pooled means of four replicates for each treatment. Embryogenic microspore% (EM%) is the percentage of dividing against the total number of microspores in a Petri dish. Since the first cell divisions of microspores from cold shock was later than those from the heat shock, only the peak values of EM% were used for comparisons. Plant regeneration frequency (PR%) is defined as the number of plants regenerated from one hundred embryoids actually transferred to the regeneration medium. The GP% was calculated as a percentage of green plants/total plants regenerated. In other words, total regenerated plants (100%) included albinos and green plants.
3. Results
3.1. The Effects of Temperature Shock on Embryogenic Development
In general, microspores isolated after cold shock exhibited much less defined features (shape and cellular organization) than those from 33˚C heat shock. The embryogenic microspores isolated after 33˚C heat shock pretreatment exhibited a well-defined, characteristic fibrillary or “star-like” structure (Figure 1(a)), with nucleus migrated more or less toward the center of cells, central vacuole fragmented by cytoplasmic strands radiated from the peri-nuclear to the subcortical cytoplasm [13] [25] [27] . Microspores from cold pretreatment showed varied and much less-defined characteristics following isolation. They only attained similar “star-like” structure 3 - 5 days in the culture for embryoid development. Well-defined embryogenic microspores (Figure 1(a)) divided rapidly to form multicellular structures within one week (Figure 1(b)), pre-embryoids (Figure 1(c)) in two weeks and mature embryoids in four weeks (Figure 1(d)). Once the mature embryoids were plated onto semi-solid medium, plantlets developed in ten days, some were albinos, and others were green plants (Figure 2). The counting of embryogenic microspores (EM), embryoids, albinos and green plants (GP) followed this developmental sequence.
A notable variation exists in the percentages of embryogenic microspores in cultures from different temperature shocks (Table 1). All lower temperature levels were able to induce microspore embryogenesis to certain extent, as compared to the control. Lower temperatures of 4˚C and 7˚C yielded higher EM% than did 10˚C and 13˚C. This trend holds true for all three genotypes studied. The optimal length of cold incubation was 6 and 10 days. For each of the three temperature levels of 4˚C, 7˚C and 10˚C, incubation of either 6 or 10 d yielded higher EM%. A lower EM% was observed from 14 d cold shock. The 2 d incubation gave rise to the lowest EM%. The 13˚C pretreatment yielded the lowest EM%, regardless of the genotype and incubation length. The 13˚C incubation of 10 or 14 d also led to higher incidences of contamination. Therefore, no further results on PR% and GP% were collected for 13˚C pretreatment. It was deemed as ineffective for embryogenic induction. Within the temperature range of 4˚C -
![]()
Figure 2. Albinos and green plants emerged from embryoids 10 days after the transfer onto semi-solid regeneration medium showing different percentages of albino vs green plants. Left: cultivar Calorwa; right: cultivar Chris.
10˚C, the lower temperature yielded higher EM%. For all three genotypes, higher EM% values were obtained from 4˚C pretreatment, as compared to all other cold shocks. However, the standard heat shock still produced the highest EM%, at 23.4%, 46.1% and 52.7% for Calorwa, Pavon 76 and Chris respectively. The best cold shock regime yielded an average of 17.3%, 34.5% and 30.7% EM for these three genotypes (Table 1). It is clear that if only measured by EM%, the heat shock remains the most effective in triggering microspore embryogenesis.
3.2. The Effects of Temperature Shock on Emryoid Yields
To offset variations in the total number of microspores in different Petri dishes, the number of embryoids was calibrated based on 1 × 104 total microspores. The numbers of embryoids from various temperature shocks generally followed the
![]()
Table 1. Embryogenic microspore% from various temperature pretreatments*.
*Each data point is a pooled mean of four replicates. **Lowercase letters denote statistical significance within the genotype and the same temperature across different incubation days; whereas capped letters denote statistical significance within the genotype and across different temperature levels. Numbers that share at least one common letter are not statistically different (p < 0.05). ***Cultures were contaminated therefore no data were available.
same pattern as the EM%: a higher EM% corresponded to a higher number of mature embryoids (Table 1 & Table 2). Among the cold shock pretreatments, 4˚C for 6 and 10 d yielded the highest numbers of embryoids, followed by these of 7˚C for 6 and 10 d, then 10˚C of the same period (Table 2). However, compared to those from 33˚C pretreatment, even the highest embryoid yields from cold pretreatment were 31%, 57% and 69% lower for Pavon 76, Calorwa and Chris respectively. For all three genotypes, the two most effective cold treatment periods remain to be 6 and 10 d. 4˚C cold shock yielded significantly higher
![]()
Table 2. Average embryoids from 1 × 104 microspores cultured following various temperature pretreatments*.
*Each data point is a pooled mean of four replicates. **Lowercase letters denote statistical significance within the genotype and the same temperature across different incubation days; whereas capped letters denote statistical significance within the genotype and across different temperature levels. Numbers that share at least one common letter are not statistically different (p < 0.05). ***Cultures were contaminated therefore no data were collected nor analyzed.
numbers of embryoids than did 7˚C, with the least numbers from 10˚C. The number of embryoids from 13˚C pretreatment, regardless of the incubation period and genotype, was the lowest. The results on embryoids once again showed that the heat shock was the most effective.
3.3. The Effects of Temperature Shock on Plant Regeneration and Green Plant%
Due to very low EM% and mature embryoids from all 13˚C pretreatments and incubation periods of 2 and 14 d, they were eliminated from regeneration experiments. The plant regeneration rates of embryoids developed from heat shock pretreatment were 35%, 61% and 63% for Calorwa, Pavon 76 and Chris, respectively. Embryoids from cold shock exhibited lower plant regeneration rates across all three temperature levels (4˚C, 7˚C and 10˚C) and two treatment periods (6 and 10 days). The differences in plant regeneration rates were statistically significant for Chris and Pavon 76, insignificant for Calorwa. Comparing the plant regeneration rates across the three cold temperature levels and two treatment periods within each genotype, no significant differences were found (Table 3).
The data on GP% seemed quite interesting. The three different genotypes
![]()
Table 3. Plant regeneration% & green plant% (in parenthesis) from transferred embryoids following several temperature pretreatments*.
*Each data point is a pooled mean of four replicates. All percentages are rounded to the nearest intact numbers. **Lowercase letters denote statistical significance in PR% within the genotype and the same incubation days across different temperature levels; whereas capped letters denote statistical significance in GP% within the genotype and the same incubation days across different temperature levels. Difference between numbers that share at least one common letter is not statistically significant (p < 0.05). ***The numbers of mature embryoids were too low to obtain meaningful data on plant regeneration% and green plant%, thus were not performed.
showed different responses to cold and heat shock. For Chris, no statistical differences in GP% were found among all cold shock pretreatments, and between any cold shock and the 33˚C heat shock. The GP% from Chris was consistently around 75%. For Pavon 76, higher GP% was obtained from cold shocks, compared with that of 33˚C. The differences in GP% among different cold shocks were statistically insignificant. Calorwa showed the most dramatic differences in GP% between the heat shock and various cold pretreatments. The GP% was only 5% for 33˚C pretreatment, but 28% - 34% for cold shocks. The GP% for the cold shocks was five folds higher than that of 33˚C heat shock (Table 3). The five-fold increase in GP% was consistent across all cold shock temperature levels with Calorwa.
3.4. Temperature Variations during Embryoid Development
None of the temperature variations during embryogenic development were found beneficial, as compared to the standard temperature of 28˚C. The control (28˚C) yielded the highest number of embryoids; the next was 24˚C and 20˚C; 16˚C produced the lowest embryoids. There was no statistical difference in embryoid yields and GP% between 24˚C (room temperature) and 28˚C (Table 4). This ranking was consistent for all three genotypes studied. Within each genotype, no significant differences in plant regeneration and GP% were observed between 28˚C and 24˚C, between 20˚C and 16˚C. However, incubation at 28˚C and 24˚C yielded higher PR% than 20˚C and 16˚C. No significant difference was found in GP% across all four temperature levels (Table 4). The temperature variations during induction culture appear to affect the number of embryoids and total plant regeneration more than GP%.
![]()
Table 4. Average total embryoid yields, plant regeneration% & green plant% from various incubation temperatures during embryogenic development*.
*Each data point is a pooled mean of four replicates. All microspores for each genotype were isolated following 33˚C pretreatment. **Letters denote statistical significance within the genotype and across different incubation temperature levels. Difference between numbers that share at least one letter is not statistically significant (p < 0.05).
4. Discussion
It is important to note that following the cold treatment to tillers, microspores appeared equivalent to the type I cells characterized by Indrianto et al. [28] , whereas those following 33˚C treatment showed a more defined “fibrillar” morphology [14] or equivalent to the type III microspores described by Indrianto et al. [28] . The difference in cell morphology exhibited by microspores following a cold and heat shock is an important marker because such difference affects the pace of embryogenic development, thus dictates corresponding strategy for doubled haploid production. In other words, variations in stress resulted in different induction frequencies, which indicate different culture efficiencies. In our experiments, it took 3 - 5 days of incubation for microspores from cold treatment to evolve into the morphology of embryogenic cells (Figure 1), characteristic of microspores immediately after isolation from the heat shock. Morphology of Figure 1 is a prelude to embryogenic development. Therefore, counting EM following a same incubation period for microspores from cold and heat shock may be misleading. We compensated this differential pace by using the peak values of EM for both types of pretreatment. Further, the differences in cellular morphology could be responsible for differences in embryoid quality, which determines subsequent plant regeneration. And finally, the diverse cellular morphology resulting from different stress treatments may offer explanations for fluctuations and inconsistency from one research laboratory to another.
Results from cold pretreatment variations indicate that only temperature levels at or below a critical point (10˚C) could induce microspore embryogenesis. Considering the temperature regime (17/25˚C night/day) under which donor plants were raised, it is no surprise that 13˚C is not effective as a stress treatment for embryogenic induction. This temperature level, only 3˚C - 4˚C below the normal night temperature for raising donor plants, poses hardly any stress to excised wheat tillers. Through our prior research, we knew that Calorwa yielded high proportion of albinos. Chris usually produced a high proportion of green plants. Pavon 76 showed moderate albino frequency. Based on our current results, the positive effects by cold shock pretreatment on GP% were more noticeable with Calorwa, which normally produced a high proportion of albinos. The GP% alone, however, is not an all-around measure of effectiveness for a particular protocol or pretreatment. One has to take into account the number of embryoids, which are affected by the number of embryogenic microspores in a Petri dish to begin with. The best measure of efficiency, in our opinion, is the total number of green plants regenerated from a given number of microspores.
One advantage of using the cold shock, however, is likely the reduction of contamination during pretreatment. At a lower temperature range of 4˚C - 10˚C, microbes reproduce much more slowly than they do at an elevated temperature of 33˚C, hence fewer contaminations occurred during embryoid cultures. In addition, cold shock offers flexibility for experiments and eases the work load when lots of samples (a real scenario in practical breeding) are ready at approximately the same time. For heat shock treatment, the time period to achieve optimal embryogenic induction is limited to a 2 - 4 hour range. Beyond that critical window, there is a significant drop in EM% (unpublished data). For cold shock, that critical period can be one to several days, which is desirable for a wider employment of this technology in plant breeding that often deals with hundreds of different breeding lines.
It is not surprising to find that temperature variations during embryoid development were not beneficial. Once the embryogenic potential was induced by a stress treatment, a critical temperature, balanced nutrients and “nursing” factors in the medium were required for optimal embryoid development, as suggested in other studies [17] [29] [30] . Within the established range of 26˚C - 28˚C, mature embryoids developed more rapidly, which shortens the time to attain doubled haploids and thus is advantageous for practical reasons. The culture temperature of 28˚C is not a contributing factor for lower GP%. Incubation of microspores at temperature lower than 24˚C led to much lower embryoid yield due to a slower pace in cell divisions and aborted subsequent differentiation.
Although data on double haploid percentages among green plants were not collected, but it is likely that temperature might also affect these numbers, since nuclear fusion during early stage of embryogenesis is at least one mechanism for chromosome doubling [6] . Varied temperatures during early embryogenic development will likely affect nuclear fusion, which results in different rates of chromosome doubling. More research is needed to study how much various temperatures during early stages of embryogenesis will affect the chromosome doubling.
5. Conclusion
In conclusion, pretreatment designed to trigger embryogenesis not only affects the number of microspores reprogrammed for embryogenesis, but also influences the quality of embryoids from these microspores. Although different genotypes responded to the same temperature pretreatment with detectable differences, some general trend concerning temperature levels was evident from the present studies. First, higher GP% was associated with the cold shock pretreatment to microspores. Thus it might be desirable to employ cold shock for genotypes that are naturally prone to albinos. Second, for germplasm that does not exhibit a higher propensity for albino regeneration, the heat shock of 33˚C is still more effective in inducing microspore embryogenesis and producing higher green plant yields. Thus, heat shock at this temperature should be employed as a default pretreatment for wheat microspore cultures. In summary, for genotypes and breeding lines that are sensitive to high temperature shock (thus consistently yielding low GP% as a result), cold shock at temperature range between 4˚C and 10˚C should be used as a viable alternative to reduce albinos among the regenerated plants.
Abbreviations
EM—embryogenic microspore;
EMB—embryoid;
PR—plant regeneration;
GP—green plant.