Prevalence and Risk Factors Associated with Tungiasis among School-Aged Children in the City of Port Gentil, Ogooué-Maritime Province, Gabon in Central Africa ()
1. Background
Tungiasis is a skin parasitosis caused by the female sand flea, Tunga penetrans (T. trimamillata in some areas) or chequered flea [1], which occurs in many countries in sub-Saharan Africa, the Caribbean and Latin America. To this end, the World Health Organization launched in 2020, “a roadmap 2021-2030”, to fight against this neglected tropical disease, in order to achieve the Sustainable Development Goals [2]. After penetration of the tick flea or T. penetrans under the skin in the feet, under the nails or between the toes of the human host, the disease is characterized by acute (multiple white, gray or yellowish papillary or nodular lesions with a brown-black colored opening in the center and peripheral erythema) and chronic inflammation in the feet with pruritic/painful lesions [3]. By being clinical and delicate, the profuse form of Tungiasis is potentially serious, as its complications are numerous, among others: septic ulcer, gangrene, abscess or even pyothrombophlebitis, tetanus and septicemia [4]. In humans, the severity of the disease is proportional to age, and it has been shown that with high prevalence rates, the 5 - 14 years age group and the elderly are the most affected, with prevalence rates as high as 53% [5]. In many villages and slums of large cities in sub-Saharan Africa (SSA), where there are still unpaved roads, large families living in extreme poverty, uncemented floors and mud brick walls inside houses, lack of improved toilets, no access to protected water sources [6], the combined prevalence of tungiasis has been shown to be 33.4% (95% CI : 27.6-39.8), while the prevalence of tungiasis was 46.5%, 44.9%, 42.0%, 37.2%, 28.1%, 22.7%, and 20.1% for Ethiopia, Cameroon, and Tanzania, Kenya, Nigeria, Rwanda, and Uganda, respectively [7]. Due to the persistent symptoms and social stigma associated with Tunga penetrans infestation, infested children find it difficult to attend school and classes [8]. In Gabon, despite the damage caused by this ectoparasite, both in urban and rural areas, epidemiological data on this infestation do not exist anywhere to our knowledge. Therefore, this study was undertaken to assess the prevalence and risk factors associated with Tungiasis in school children in the city of Port Gentil, an endemic area, in the province of Ogooué-Maritime, Gabon.
2. Materials and Methods
2.1. Study Area, Type and Period
This prospective and cross-sectional study was conducted from 22 May to 18 August 2022, in the city of Port Gentil, in the province of Ogooué-Maritime, Gabon. Port Gentil is the capital of the province of Ogooué-Maritime, the second largest city in Gabon in terms of population (just under 137,000) according to the 2013 national census. Located on Mandji Island, 144 km southwest of Libreville, Gabon’s political capital, Port Gentil enjoys a tropical climate, punctuated by a “dry” and a “rainy” season. The city is bordered by beaches. The geographical coordinates of the city indicate a latitude of 0˚43' South to 0˚00' South and a longitude of 8˚46' East to 8˚30' East [9]. With a tropical climate, there is a hot and rainy period from October to May and a relatively cool and dry period June to September in Port-Gentil [10]. Among the districts of Port Gentil, namely Matanda, Balise and Grand Village, Sibi, Salsa, Fatima, and Tournand Sindara, two popular districts, namely and Matanda, Sidara were selected for this study.
2.2. Study Population
The study population consisted of all school-aged children enrolled in primary and secondary schools in the city of Port Gentil in 2022, residing in the two selected neighborhoods. The total number of children in the study was 637, of which 303 were male and 334 were female.
2.3. Inclusion Criteria
Only children in the two wards of Matanda and Tournand Sindara who were willing to participate and whose parents/guardians had agreed to be interviewed face-to-face in accordance with written consent were enrolled in the study.
2.4. Exclusion Criteria
Children who refused to participate or whose parents/guardians did not respond to our request were excluded from the study.
2.5. Determination of Sample Size
To estimate the prevalence and correlates of Tunga penetrans infestation in two neighborhoods in the city of Port-Gentil, the sample size for the study was determined using the single proportion population formula as done elsewhere [11] by positing the formula:
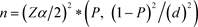
In it, n represents the number of the sample size, Zα/2 is the standard normal deviation (1.96) corresponding to a 95% confidence interval (CI), P is the prevalence of Tunga penetrans infection. In the absence of p-values obtained elsewhere or in previous studies in the city of Port-Gentil, this P-value was taken as 50%). d is considered the precision/marginal error (d = 0.05) or 5%. Initially, the sample size determined for the study was 580. As it has been applied in two studies elsewhere, errors resulting from the probability of noncompliance or dropout were minimized, the sample size was increased by 10% [12] [13]. Finally, the sample size used in the study was 637 children.
2.6. Sampling Techniques
Using a purposive sampling method due to the large number of children and the crowded conditions in which they lived, two popular and under-integrated neighborhoods were selected from among the others in the city of Port Gentil. The number of children in each neighborhood was based on the total number of children in each neighborhood divided by the total number of children in the selected neighborhoods. The results were decided by multiplying the calculated sample size. As has been done in previous studies, the selection of study participants was stratified. Based on their grade level (grades 1 - 5), the children were first stratified into eight strata within the selected neighborhoods. Then, using the proportional allocation technique from each stratum, the children were allocated by neighborhood and by grade level [14] [15].
2.7. Data Collection Instruments
As the city of Port-Gentil is cosmopolitan, a questionnaire was prepared in French according to the objectives of the study and the local situation, to collect socio-demographic, environmental and behavioral factors associated with Tunga penetrans infestation. Each investigator was asked to interview the children or their parents/guardians and to complete the questionnaire.
2.8. Data Collection
As usual for a field survey, appropriate instructions were given to the interviewers to collect the data for this study. To see the existence of Tungiasis (presence of T. penetrans encrusted in the legs, feet, hands and arms, the investigators first proceeded as it has been done elsewhere, to a physical examination of the children, based on characteristics of jigger infestation, found using the classification of Fortaleza. That is, presence of a dark, itchy spot on the skin, or distinct craters like wounds in the skin [16]. Then, using a standardized questionnaire containing socio-demographic, economic, environmental and behavioral factors, as well as conditions related to the disease, the investigators interviewed the children’s parents or guardians. Finally, other relevant conditions, such as the sanitation of the children’s living area, were observed and assessed using a structured checklist [17].
2.9. Study Variables
The dependent variable was the detection of T. penetrans in the skin of any school-aged child at the time of data collection. In this study, any child found to be Tungiasis-positive during a physical examination was considered to be positive for Tungus by washing the child’s feet and hands with soap and water, followed by careful observation of these areas. Socio-demographic factors, such as neighbourhood of residence, gender, age, educational level of the child, ethnic origin of the child, occupation of the parents, educational level of the fathers and mothers, environmental factors (type of soil inside the house, cemented or earth/sand) and behavioural factors (playing in the sand barefoot, Source of water consumed, wearing of shoes, promiscuity with dogs or cats in the house, wearing of shoes, housing conditions of the family, family standard of living, number of people living in the house, were the independent variables.
NB: Ethnicity refers to the cultural background of the child’s parents participating in the study.
2.10. Ethical Considerations
The objectives and procedures of this study were explained and written informed consent was obtained from the parents or guardians of the children who met the inclusion criteria and participated in this study. The prevalence questions of the study were approved and validated by members of the ethics group of the Department of Biology of the Masuku University of Science and Technology in Franceville, Gabon. Data were recorded during sample collection. The anonymity of the study participants was ensured through the use of coded numbers and the information obtained during the study was kept secret. Paper data was kept in a locked cabinet in a confidential manner and computer data was secured by passwords. With the exception of the team members, no one had permission to access the children’s data.
2.11. Statistical Analyses of the Data
The data collected was coded, entered into a Microsoft Excel spreadsheet, cleaned and then analysed using R software in its R Commander interface for two types of analysis. While a descriptive analysis was chosen for the socio-demographic characteristics and prevalence of Tungiasis in the participants, a multivariate logistic regression analysis according to the usual behavioural and environmental characteristics was performed and the variables in this analysis reported odds ratios and their 95% confidence intervals. And the results were statistically significant at p < 0.05 and the identified variables were considered as risk factors.
3. Results
3.1. Prevalence of Tungiasis in School-Age Children (n = 637) in the Study
In the present study, a total of 637 children were registered in the working-class and disadvantaged neighborhoods of the city of Port-Gentil. All of them (100%) were willing to participate in this study, and their parents/guardians responded fully to the questionnaire to which they were subjected. With a mean age of 12.73 years and a standard deviation of 5.44, the overall prevalence of Tungiasis in the participants of this study, was 37.99%, (95% CI: [0.34- 0.41]), (n = 242), against 62.01% (n = 395) who were negative (Figure 1).
![]()
Figure 1. Prevalence of Tungiasis among school-aged children (n = 637) in the study.
3.2. Socio-Demographic Characteristics Associated with the Prevalence of Tunga penetrans in School-Aged Children (n = 637) in the Study
After exploring the possible associations between the socio-demographic characteristics of the study population (n = 637) and the prevalence of Tunga penetrans infestation using univariate analysis, the variables were fitted in a multivariate logistic regression model. Results indicated that, children in the age group of 5 - 9 years (Adjusted Odds Ratio = 9.27; 95% CI: [6.66 - 12.91]) and 10 - 14 years (Adjusted Odds Ratio = 0.16; 95% CI: [0.10 - 0.25]), Similarly compared to other ethnicities, children from Eshira ethnicity had 7.46 times higher risk of tungus infestation (Adjusted Odds Ratio = 7.46; 95% CI: [2.97 - 18.76]), In addition, compared with secondary school children, ,primary school children had a 0.008 times higher risk of tungus infestation (Adjusted Odds Ratio = 0.008; 95% CI: [0.006 - 0.011]), Children from households, whose Father/Guardian (primary) education level was primary, also had a 0.22 times higher risk of tungiasis infestation (Adjusted Odds Ratio = 0.22; 95% CI: [0.13 - 0.35]), finally, children whose mother’s or guardian’s status was that of housewife, were significantly exposed to Tungiasis infestation (Adjusted Odds Ratio = 4.89; 95% CI: [3.51 - 6.79]) (Table 1).
![]()
![]()
Table 1. Univariate and multivariate analyses of the prevalence of Tungiasis, according to the socio-demographic characteristics of the study population (n = 637).
* = Significant test.
3.3. Usual Behavioural and Environmental Characteristics Associated with the Prevalence of Tungiasis in School-Aged Children (n = 637) in the Study
To test the association between the usual behavioural and environmental characteristics of the school-aged children (n = 637) in the study and exposure to Tungiasis, a crude analysis of the variables was performed. Using multivariate logistic regression, it was found that being around dogs, cats or other animals in the home (Adjusted Odds Ratio =, 38.30; 95% CI: [22.13 - 66.26]), wearing the shoes a few times (Adjusted Odds Ratio = 0. 02; 95% CI [0.01 - 0.03]), walking sometimes barefoot, on land or sand (Adjusted Odds Ratio = 0.28; 95% CI: [0.18 - 0.42]), having other sources of water consumed than tap water (Adjusted Odds Ratio = 0. 25; 95% CI: [[0.16 - 037]), living in a dwelling with a soil or sand floor (Adjusted Odds Ratio = 6.8; 95% CI: [4.58 - 10.09]), having average housing conditions (Adjusted Odds Ratio = 46.8; 95% CI [24. 29 - 90.16]), and the number of people living in the family was greater than or equal to 6 (Adjusted Odds Ratio = 0.1; 95% CI [0.07 - 0.13]), had a very high probability of being infested by Tunga penetrans. These risk factors were significantly associated with Tunga penetrans infestation (Table 2).
![]()
Table 2. Univariate analysis and multivariate logistic regression of Tungiasis prevalence according to behavioural and environmental background characteristics in school-aged children (n = 637) in the study.
*: significant test.
4. Discussion
Very common in developing countries and producing a high global disease burden, Tunga penetrans is an ectoparasite of both humans and animals. To design, plan and evaluate appropriate intervention strategies against this zoonosis, knowledge of the epidemiology, transmission, distribution and extent of this disease and associated risk factors in children is very important [18]. The main objective of the present study was to determine the prevalence of tungus and associated risk factors in school-aged children in the city of Port Gentil, Ogooué-Maritime province, Gabon. The study indicated an overall prevalence of 37.99% for this disease. Although higher than the results obtained in Kenya (25%) [18], and Ethiopia (28.3%) [17], this result is considerably lower than those obtained in children aged 5-14 years in the rural Woreda of Mettu [5] and in the Densho district [16] in Ethiopia, where studies obtained prevalences of 52.3% and 58% respectively. Similarly, in resource-poor rural and urban areas of some countries, prevalences of up to 45% have been reported [19] [20] [21] [22]. However, the result of the present study is almost comparable to that obtained in southern Ethiopia (34.87%) [23]. The variability in Tungiasis prevalences may be due to many parameters such as, different study schedules, seasons and years, sampling of study participants, socio-demographic factors, personal hygiene practices, differences in education levels or awareness of parasite transmission and prevention, in the study areas. Similarly, bioclimatic conditions may play a role in the reported differences in prevalence. The univariate and multivariate analysis models of Tungiasis prevalence, according to the socio-demographic characteristics of the study population (n = 637), revealed six determinants such as the age range of 5 - 9 years and 10 - 14 years, children of Eshira ethnicity, the child’s primary education level, Father’s/Guardian’s (primary) education level, housewife status were significantly associated with the prevalence of Tungiasis, ethnicity, father’s/guardian’s education level, children’s education level, and parents’/guardians’ occupational status, which were significantly associated with the prevalence of Tunga penetrans infestation. Firstly, the prevalence of Tunga penetrans infestation was significantly associated with two age groups of 5 - 9 years and 10 - 14 years, representing a class of young children [24]. This result is in agreement with those obtained in studies conducted elsewhere, which indicated that only children aged 5 - 14 years and the elderly were most affected by tungiasis, with prevalence rates of up to 53% [5] [17]. This result indicates the “infantile” nature of Tungiasis in the study area since, with a lower level of health education than secondary school children, the daily activities of children in these age groups expose them to a less hygienic environment, and thus to many risk factors for infections by different parasites [24], including infestation by Tunga penetrans, the cause of Tungiasis [6]. As rural-urban migration is a factor of economic immigration for certain populations other than the indigenous (Myenes), our study indicated a prevalence of Tungiasis associated with children of Eshira ethnic origin. This corroborates with the fact that, as these populations come from other provinces of Gabon, only the often overcrowded peri-urban neighbourhoods of the city of Port-Gentil, and lacking adequate facilities, are where they live. Children whose fathers/guardians had a low level of education (primary school) or those whose mother’s/guardian’s socially inferior professional status (housewife) were likely to be infested with Tungiasis This demonstrates that, although it may vary according to the social and cultural norms of each region or country, the upbringing of parents or guardians in a family, their social position and their interest in the welfare and education of children, gives them skills to advocate for or against traditional practices that may impact on children’s health [25]. In contrast to studies conducted elsewhere, the results of our study revealed that the prevalence of Tunga penetrans infestation was significantly associated with children of primary education level. This is consistent with the fact that as long as children’s living conditions, sanitation and hygiene are precarious, they remain exposed to neglected tropical diseases, in this case parasitic diseases [26].
In univariate and multivariate analysis of the prevalence of tungiasis, according to the usual behavioural and environmental characteristics of the school-aged children in the study, the results indicated numerous risk factors for exposure to tungiasis. These included being in close proximity to dogs, cats or other domestic animals, sometimes walking barefoot on dirt or sand, living in a house with a dirt or sand floor, having water from a source other than the tap, having average housing conditions, and the number of people living in the family was greater than or equal to 6. Indeed, parasitic infestation was more frequent in children who had close contact with dogs, cats or other pets (Adjusted Odds Ratio = 38.30; 95% CI: [22.13 - 66.26]), than those who did not. This result, in agreement with those found in studies conducted elsewhere, may be explained by the fact that domestic cats and ruminants harbour high intensities of sand fleas in endemic communities [27]. And that a wide range of domestic and peri-domestic animals, such as dogs, cats, pigs and rats constitute an animal reservoir for tungiasis [28]. Close contact between these important pets and humans promotes exposure and the risk of infection is very high [29]. The chances of children who wore the shoes a few times (Adjusted Odds Ratio = 0.02; 95% CI [0.01 - 0.03]), were 0.02 times more likely to acquire Tungiasis, than those who always wore them. Similarly, those who sometimes walked barefoot on dirt or sand (Adjusted Odds Ratio = 0.28; 95% CI: [0.18 - 0.42]) were more likely to be infected with the parasite than those who did not do so at all. This can be explained by the fact that the prevention of this disease also relies on the wearing of closed shoes, which is the primary means of foot protection [30], and the lack of this, exposes children to infestation. According to the present study, children living in families that used other sources of drinking water were also 0.25 times more likely to contract tungus than those who consumed tap water, (Adjusted Odds Ratio = 0.25; 95% CI: [0.16 - 037]). This can be justified by the fact that drinking tap water, which is safe to drink, ensures good health for the child [31]. In many poor and deprived villages and neighbourhoods often clustered outside the major cities in sub-Saharan Africa (SSA), where there are still unpaved roads, large families living in extreme poverty, uncemented floors and mud-brick walls inside the houses, lack of improved toilets, no access to protected water sources and more wild animals [32] [33]. In the present study, children who lived in dwellings with earth or sand floors (Adjusted Odds Ratio = 6.8; 95% CI: [4.58 - 10.09]), or who had average housing conditions (Adjusted Odds Ratio = 46.8; 95% CI [24.29 - 90.16]), were 6.8 and 46.8 times more likely to be infested by T. penetrans than those who lived in dwellings with cement floors and good housing conditions. This result is similar to that obtained by studies conducted elsewhere, which indicated that the dust generated by the sand and soil creates ideal conditions for the development cycle of the Tungiasis parasite outside the final host, in the cracks of the soil. Therefore, a house with a cemented or tiled floor would reduce the prevalence of tungus [34]. Furthermore, the results of the current study revealed that children living in families with 6 or more persons were 1.86 times more likely to contract tungiasis than children from small families (Adjusted Odds Ratio = 0.1; 95% CI [0.07 - 0.13]). This may be justified by the fact that in large families, by escaping parental control, children are able to multiply their play areas, increasing the chances of being infested by T. penetrans. Although it has been reported in one study that inadequate childcare increases the risk of stunting [35], children in large families may not receive special care from parents. As a result, they are exposed to a very high risk of parasitic diseases including Tungiasis. This is the case of helminthiasis, a real public health problem. They cause chronic inflammatory disorders such as chronic anaemia, stunted growth, protein-calorie malnutrition, fatigue, poor cognitive performance, reduced long-term survival, reduced physical fitness and reduced school attendance in school-age children [36] [37].
5. Conclusion
Age, ethnic origin, parents’/guardians’ education level, children’s schooling level, and parents’/guardians’ professional status, promiscuity with dogs, cats or other animals, walking sometimes barefoot, on earth or sand, having other sources of water consumed than the tap, living in a house with soil or sand inside, having average housing conditions, and the family having 6 or more people living in it, were all found to be related to tungiasis in the study population. The results of this study showed that the high incidence of tungiasis was clearly associated with sociodemographic, environmental and behavioral variables. These results may guide health authorities in Gabon in the control and prevention of T. penetrans infestation. They should focus on infested areas to improve sanitation in order to reduce the prevalence of tungiasis and the burden of parasitic diseases among school-aged children and their families in the city of Port-Gentil.
Limitations of the Study
In spite of the contributions made, the present study has some limitations that deserve to be highlighted, in order to take them into account in future studies. Within the conceptual framework of the study, we identified a number of upstream factors (life context of the children and their parents/guardians) and risk factors that may influence Tunga penetrans infestation. First, this study was limited to school-aged children in two poor and disadvantaged neighborhoods of the city of Port-Gentil in Gabon. Extending this study to children residing in other poor neighborhoods of the city could be useful to obtain a broader picture of the prevalence of tungus in the city of Port-Gentil. Second, due to the unavailability of resources, we were unable to obtain a magnifying lens, an equipment that could allow to distinguish skin lesions caused by T. penetrans without being hampered by superficial skin lesions. However, to the best of our knowledge, this study is the first approach to determine the prevalence and risk factors associated with infestation in school-aged children in the city of Port Gentil, Gabon.
Acknowledgements
The authors would like to thank all respondents and data collectors who contributed to this study. They would also like to thank the Department of Biology of the University of Science and Technology of Masuku in Franceville, Gabon for its support.
Data Availability
Not applicable. However, raw data can be obtained from the corresponding author upon kind request.
Author Contributions
TNM, CSO, CB, AN, and PM were responsible for the study design. TNM, PM, and CB were responsible for the conduct of the study. HMK, BAPP, AN, TNM, PM, CB, and UZ participated in the analysis and interpretation of the data. Physical examination and participant interviews were performed by UZ, HMK, BAPP, and CSO. Finally, the manuscript was written and edited by TNM, CB, AN, and PM. All authors read and approved the final version of the manuscript for publication.
Abbreviations
OR: Crude Odds ratio
T. penetrans: Tunga penetrans
CI: Confidence interval
N: Number of participants
(%): Percentage