Pleural Effusion Revealing Bone Ewing Sarcoma: About Three Cases ()
1. Introduction
Bone Ewing’s sarcoma (ES) is a fairly rare tumor, accounting for approximately 1% of childhood malignancies and approximately 5% - 15% of primary bone malignancies [1]. The average age group for Ewing’s sarcoma is 13 years with a predilection for men. It belongs to a family of tumors which includes the Askin tumor and the primary peripheral neuroectodermal tumor (PPNET) [2]. The primary bone lesion is usually in the femur, pelvic bone, humerus, fibula, collarbone, or tibia. The ribs, scapula, and vertebrae are infrequent sites [3]. We report the case of three children who presented a pleural effusion revealing Ewing sarcoma affecting the ribs.
2. Observation 1
This is a 10-year-old male child from a non-consanguineous marriage, the eldest of two siblings, with a two-year history of bacterial pneumonia previously treated with antibiotics. Admitted for the management of left lateral thoracic pain dating back 2 weeks before admission with exertional dyspnea and fits of dry cough without hemoptysis, all developing in the context of asthenia, apyrexia, and weight loss estimated at 4 kg. Clinical examination found the child hemodynamically and respiratory stable at 100% SaO2 in room air. The pleuropulmonary examination revealed a syndrome of left fluid effusion. The cardiovascular examination was otherwise unremarkable. Paraclinically, the thorax X-ray revealed a very abundant left pleurisy (Figure 1), the thoracic ultrasound found encysted pleurisy with a liquefied hematoma, and the puncture was in favor of a non-coagulating serohematical fluid, with no absence of bacterial flora. The biological assessment objectified the NFS hyperleukocytosis at 19,800/yl, predominantly PNN at 12,350/yl, the CRP was high at 250 mg/l, and physiological assessment: IDR and BK sputum were negative. Surgical exploration was in favor of pleuropneumopathy with multiple aspects of the septum and serohematic effusion with granulation of the visceral and parietal pleura, completed by a pleural biopsy, which was in favor of granulomatous pleuritis without giant cells or necrosis. Caseous with the absence of signs of malignancy suggesting in the first place a tuberculous origin. The child was put on antitibacillary treatment for six months, however, the evolution was marked by the absence of clinico-radiological improvement (reappearance of lateral thoracic pain with asthenia and dyspnea on exertion).
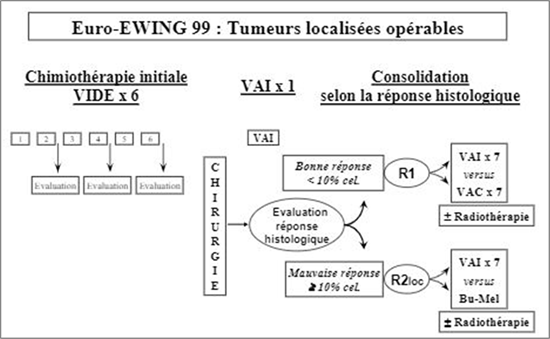
Thus, the thoracic scanner revealed a well-limited bilobed left tumor process which was enhanced heterogeneously after contrast, delimiting more marked areas of necrosis at the level of its lower pole, measuring 198/112 mm, achieving a significant mass effect on the ipsilateral lung, and the mediastinal structures deviated to the right with costal lysis of the posterior arch of the left 10th rib and the presence of supraclavicular and laterocervical and mediastinal lymphadenopathy (Figure 2). The CT appearance and ultrasound-guided biopsy of the pleural mass were in favor of bone Ewing sarcoma. The extension assessment (marrow and BOM) showed the absence of medullary infiltration, and the tumor lysis assessment showed an increase in LDH to 1993 U/L, uric acid was high at 54 mg/L, l blood ionogram was without abnormalities. From a therapeutic standpoint, neoadjuvant chemotherapy was started according to the EuroEwing 99 protocol: VIDE (combination of doxorubicin, etoposide, ifosfamide, and vincristine) (Annex 1) with a pre-chemotherapy assessment showing no particularity. The patient underwent total resection of the tumor and the posterior arch of the 10th rib, then post-operatory radio-chemotherapy with good clinical and radiological progress after 6 months of follow-up.
![]()
Figure 1. Frontal chest X-ray revealing a very abundant left pleurisy.
![]()
Figure 2. CT scan in favor of a well-limited bilobed left tumor process containing areas of necrosis with costal lysis of the posterior arch of the 10th left rib.
3. Observation 2
This is a 13-year-old girl from a non-consanguineous marriage, the eldest of two siblings, with no notable medical history. Admitted for management of left basal thoracic pain dating back 2 weeks before admission with fever and exertional dyspnea, all developing in the context of asthenia, apyrexia, and unstated weight loss. Clinical examination found a respiratory stable child with a respiratory rate of 20 cpm, 98% SaO2 in room air, tachycardium at 127 bpm, normal strain at 130/90 mmHg with slightly discolored conjunctivae. The pleuropulmonary examination revealed a syndrome of left fluid effusion. The cardiovascular examination was otherwise unremarkable. Para clinically, the thorax X-ray revealed a very abundant left pleurisy. The CT appearance revealed a voluminous tumor process centered on the left pleural space, locally advanced pleural effusion and with costal lysis of the left 6th rib (Figure 3). The biopsy of the mass was in favor of Ewing’s sarcoma.
![]()
Figure 3. CT showing a mass lung tumor lower left lobe with pleural effusion and costal lysis homolateral of the 6th dimension.
The extension assessment (marrow and BOM) showed the absence of bone marrow infiltration, tumor lysis assessment and blood ionogram were without abnormalities. From a therapeutic standpoint, neoadjuvant chemotherapy was started according to the EuroEwing 99 protocol: VIDE (combination of doxorubicin, etoposide, ifosfamide, vincristine) (Annex 1) with a prechemotherapy assessment showing no particularity. The patient underwent adjuvant surgery with resection of the posterior arch of the sixth rib adherent to the mass and complete resection of the tumor in one piece. The anatomopathological study of surgical speci2 & men confirmed the small round cell tumor of the PNET group (Ewing’s sarcoma). The evolution was favorable and the patient is under postoperative chemotherapy and radiotherapy following the same protocol mentioned above.
4. Observation 3
This is a 13-year-old girl from a consanguineous marriage, the youngest of three siblings, with no notable pathological history. Admitted for a dry cough with left basal thoracic pain dating back one month before admission, all progressing in a context of apyrexia and deterioration of the general condition. Clinical examination found a child hemodynamically and respiratory stable, 100% SaO2 in ambient air. The pleuropulmonary examination revealed a syndrome of left fluid effusion. The remainder of the physical examination was otherwise unremarkable.
Paraclinically, the chest X-ray revealed a basic left thoracic opacity (Figure 4), the chest scanner finds a large left pleural mass measuring 120 × 97 × 127 mm, locally advanced pleural effusion and with costal lysis of the anterior arch of the left 7th rib (Figure 5). The biopsy of the mass was in favor of Ewing’s sarcoma.
The extension assessment (marrow and BOM) showed the absence of medullary infiltration, the tumor lysis assessment was without abnormalities, scintigraphy revealed a diffuse and moderate hyper fixation from K3 to left K8 with costal micro focus in K7 left. On the therapeutic level, neoadjuvant chemotherapy
![]()
Figure 4. Frontal chest X-ray revealing a basi left thoracic opacity.
![]()
Figure 5. CT scan shows a large necrotic left pleural mass polylobed tissue with an irregular and discreetly eroded aspect of the 7th rib.
was started according to the EuroEwing 99 protocol: VIDE (Annex 1). The patient underwent adjuvant surgery with resection of the anterior arch of the seventh rib adherent to the mass and complete resection of the tumor in one piece. The anatomopathological study of the surgical specimen confirmed the small round cell tumor of the PNET group. The patient received postoperative chemotherapy and radiotherapy courses according to the same protocol with good clinical and radiological progress after one year of follow-up.
5. Discussion
Musculoskeletal tumors are rare, but responsible for significant morbidity and mortality in children. The diagnosis of Ewing’s sarcoma rests on a bundle of arguments. It is a synthesis of interrogation, clinical examination, imaging, laboratory examinations, and finally pathology. The thoracic manifestations are dominated by chest pain and dyspnea; patients usually present with a painful mass on the chest wall. The presentation of a pleural effusion, as in our case, is an unusual result [4] [5]. Ewing sarcoma of the ribs tends to spread inward towards the chest cavity and may therefore manifest as an extrapleural mass. The radiographic appearance of the ES of the rib cage is variable. The affected rib is predominantly lytic in most cases, but mixed lytic-sclerotic and even predominantly sclerotic patterns are also encountered [6]. The diagnosis of the tumor is essentially histological, preferably with a surgical bone biopsy carried out in a referral center laboratory capable of carrying out the necessary cytogenetic and molecular biology examinations on the tumor. Optimal treatment is achieved with multidrug chemotherapy and subsequent rib resection and/or radiation therapy. The probability of survival without recurrence at five years is with current treatments, 70% in localized form and 30% in metastatic form.
6. Conclusion
Primary malignant rib tumors in children are very rare and mainly represented by SE/PNETs belonging to the Ewing tumor family. The certainty of the diagnosis is posed by the histological examination, based on clinical and especially radiological data. Ewing’s tumor is a fascinating reflection of the evolution of cancer thinking. Initially, crude therapeutic strategies have gradually become more precise thanks to increased knowledge of the natural course of the disease and the factors predicting this course.
Annex 1
EuroEwing 99 protocol: VIDE (combination of doxorubicin, etoposide, ifosfamide, vincristine).