Experimental and Theoretical Investigations on Copper Corrosion Inhibition by Cefixime Drug in 1M HNO3 Solution ()
1. Introduction
Copper [1] [2] is one of the most used metals for several engineering and industrial applications including electricity and electronics, communications, pipelines for domestic and industrial water utilities, fabrication of heat exchanger tubes, and cooling water systems due to its excellent properties. Thus, copper and its alloys are deployed in various environments containing acids, alkalis, and salt solutions where they undergo corrosive attacks although the copper is relatively a noble metal [3]. Some ways of corrosion prevention are the use of more resistant alloys, protective coating, and the addition of inhibitors to the corrosive environment [4] [5]. Among numerous ways to protect the copper from acid corrosion, the most effective and practical method [6] [7] to slow down corrosion processes, is the addition of organic corrosion inhibitors to the metal’s environment. It is reported [8] [9] that the adsorption of these compounds is influenced by the electronic structure of inhibiting molecules, the steric factor, aromaticity, electron density at the donor site, the functional group, and also the polarizability of the group. Previous studies revealed that heterocyclic compounds [10] are employed as corrosion inhibitors because of the presence of various adsorption centers (O, N, S, P, and π electrons) which can help to form complexes with metal ions. Unfortunately, the use of some organic chemical inhibitors is limited by diverse reasons especially their costly synthesis, poor biodegradability, toxic and hazardous for human beings and the environment as well. For this purpose, natural products of plant origin or drugs are a better choice due to the fact that they are environmentally benign and contain incredibly rich sources of naturally synthesized organic compounds among which most of them are known to have inhibitive action. Plant extracts are often insoluble in aqueous media and extraction efficiencies are mainly low. The choice of some drugs used as corrosion inhibitors is based on the following justifications: 1) drugs are mainly soluble in aqueous media; 2) drug molecules contain oxygen, nitrogen and sulphur as active centers; 3) drugs are reportedly environmentally friendly and important in biological reactions; and 4) drugs including β-lactam antibiotics, quinolones, antifungal, and cephalosporins can be easily produced and purified [11] [12] [13] [14]. The aim of the present paper is to highlight the relationship between the calculated quantum chemical parameters and the experimentally determined inhibition efficiency of cefixime drug (Scheme 1) against copper corrosion in 1M HNO3 medium. This can be achieved by computing the most relevant electronic properties of the studied compound including EHOMO, ELUMO, energy Gap (ΔE), dipole moment (μ), electronegativity (χ), global hardness (η), fraction of electrons transferred (ΔN) and charges (δ) on atoms.
2. Experimental
2.1. Copper Samples
The copper specimens were in form of rod measuring 10 mm in length and 2.2 mm of diameter. They were cut in commercial copper of purity 95%.
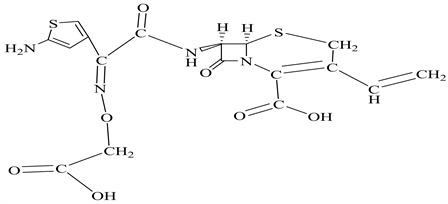
Scheme 1. Chemical structure of cefixime.
2.2. Solutions
Cefixime of analytical grade was acquired from Sigma-Aldrich. Analytical grade 65% nitric acid solution from Sigma-Aldrich Chemicals was used to prepare the corrosive aqueous solution. The solution was prepared by dilution of the commercial nitric acid solution using double distilled water. The blank was a 1M HNO3 solution. Solutions of cefixime with concentrations in the range of 0.02 mM to 2 mM were prepared. Acetone of purity 99.5% was also purchased from Sigma-Aldrich Chemicals.
2.3. Weight Loss Technique
Prior to all measurements, the copper samples were mechanically abraded with different grade emery papers (1/0, 2/0, 3/0, 4/0, 5/0, and 6/0). The specimens were washed thoroughly with double distilled water, degreased and rinsed with acetone and dried in a moisture-free desiccator. Weight loss measurements were carried out in a beaker of 100 mL capacity containing 50 mL of the test solution. The immersion time for weight loss was 1 h at a given temperature. In order to get good reproducible data, parallel triplicate experiments were performed accurately and the mean value of the weight loss was used to assess the corrosion rate (CR), the degree of surface coverage (θ) and the inhibition efficiency (IE) using Equations (1)-(3) respectively:
(1)
(2)
(3)
where CR0 and CR (expressed in mg·h−1·cm−2) are respectively the corrosion rate without and with cefixime, ∆m is the weight loss, S is the total surface of the copper specimen and t is the immersion time.
2.4. Quantum Chemical Approach
The present quantum chemistry calculations were performed with Gaussian 09 series of program package [15]. For this purpose, we used Becke’s three parameter exchange functional along with the Lee-Yang-Parr non-local correlation functional (B3LYP) [16] using 6-31G (d, p) basis Set. Figure 1 shows the optimized chemical structure of cefixime.
In order to explore the theoretical-experimental consistency, quantum chemical calculations were performed and the electronic properties resulting from this study were analyzed through global reactivity parameters and local reactivity parameters.
2.4.1. Global Reactivity Parameters
Recently, DFT has become a very powerful technique to probe the inhibitor/surface interaction and to analyze experimental data by providing insights into the chemical reactivity and selectivity [17] [18]. So, the molecular descriptors namely the highest occupied molecular orbital energy (EHOMO), the lowest unoccupied molecular orbital energy (ELUMO), the energy gap (ΔΕ = ΕLUMO − ΕHOMO) and the dipole moment (μ) were calculated. The reactivity descriptors, including ionization energy (I), electron affinity (A), electronegativity (χ), hardness (η), softness (S), the fraction (ΔN) of electrons transferred and the electrophilicity index (ω) were also calculated. According to Koopman’s theorem [19], the ionization energy (I) can be approximated as the negative of the highest occupied molecular orbital (HOMO) energy (Equation (4)):
(4)
The negative of the lowest unoccupied molecular orbital (LUMO) energy is related to the electron affinity A and is obtained from Equation (5):
(5)
The electronegativity [20] is obtained using the ionization energy I and the electron affinity A by Equation (6):
(6)
The hardness which is the reciprocal of the electronegativity was obtained by Equation (7):
![]()
Figure 1. Optimized structure of cefixime calculated by B3LYP/6-31G (d, p).
(7)
when the organic molecule is in contact with the metal, electrons flow from the system with lower electronegativity to that of higher electronegativity until the chemical potential becomes equal. The fraction of electrons transferred, ΔN, was estimated according to Equation (8) [21]:
(8)
The values of experimental work function
[22] and hardness
[23] (since for bulk metallic atoms I = A) were considered to calculate ΔN.
The global electrophilicity index, introduced by Parr [24] is given by Equation (9):
(9)
2.4.2. Local Reactivity Parameters
The local selectivity of a corrosion inhibitor [25] is generally assessed using Fukui functions which enable us to distinguish each part of the studied compound on the basis of its chemical behavior due to different substituent functional groups. The Fukui function is defined as the derivative of the electronic density ρ(r) with respect to the number N of electrons:
(10)
The condensed Fukui functions provide information about atoms in a molecule that have a tendency to either donate (nucleophilic character) or accept (electrophilic character) an electron or a pair of electrons [26]. The nucleophilic and electrophilic Fukui function for an atom k [27] can be computed using a finite difference approximation as seen in Equations (11)-(12) respectively:
for nucleophilic attack (11)
for electrophilic attack (12)
where
,
and
are the charges of the atoms on the systems with (N+ 1), N and N − 1 electrons respectively.
Recently, it has been reported [28] that a new descriptor has been introduced [29] [30] which allows the determination of individual sites within the molecule with particular behaviors. A mathematical analysis reveals that dual descriptor is a more accurate tool than nucleophilic and electrophilic Fukui functions [31]. This descriptor is defined through Equation (13):
(13)
The condensed form [29] of the dual descriptor is given by Equation (14):
(14)
when
, the process is driven by a nucleophilic attack and atom k acts as an electrophile; conversely, when,
the process is driven by an electrophilic attack on atom k acts as a nucleophile. The dual descriptor
is defined within the range [−1; 1], what really facilitates local reactivity interpretation [31].
3. Results and Discussion
3.1. Weight Loss Measurements
3.1.1. Effect of Inhibitor Concentration and Temperature on Corrosion Rate
The corrosion rate curves of copper without and with the addition of cefixime in 1M HNO3 at different temperatures are presented in Figure 2. This figure indicates that corrosion rate of copper in the studied medium, increases with increasing temperature. But this evolution is moderated when the concentration of the studied inhibitor increases, revealing the effectiveness of the molecule as a corrosion inhibitor for copper in 1M HNO3. These results could be interpreted as the formation of a film barrier which isolates copper from its aggressive environment [32] [33].
Figure 3 displays the evolution of inhibition efficiency versus temperature. The inhibition efficiency reaches a value of 91.07% for the concentration of 2 mM at 298 K (Figure 3). The inhibitive action of the studied drug may be due mainly to the presence of heteroatoms such as oxygen, nitrogen, sulfur and aromatic rings with π-bonds in cefixime structure and its ability with these heteroatoms to create a protective film on the metal corroding surface [34].
As shown in Figure 3, inhibition efficiency decreases when the temperature rises. This occurs due to the desorption effect of the inhibitor molecules [35].
![]()
Figure 2. Evolution of corrosion rate with temperature for different concentrations of cefixime.
![]()
Figure 3. Inhibition efficiency versus temperature for different concentrations of cefixime.
Moreover, decrease in inhibition efficiency with temperature rise can also be attributed to increased solubility of the protective film and/or any reaction product precipitated on the surface of the metal [36]. As a result, the metal surface becomes more accessible to corrosive attack.
3.1.2. Adsorption Isotherms and Thermodynamic Functions
The basic information on the interaction between the inhibitor and the metal can be provided by the adsorption isotherm. The adsorption isotherms tested in this work are the models of Langmuir, Temkin, Freundlich, El-Awady and Flory Huggins. By fitting the degree of surface coverage (θ) and the inhibitor concentration (Figure 4), the best adsorption isotherm obtained graphically is Langmuir adsorption isotherm with a strong correlation (R2 > 0.999) and the slopes of the straight lines are close to unity.
The obtained Langmuir adsorption parameters for different temperatures are presented in Table 1.
Assumptions of Langmuir relate the concentration of the adsorbate in the bulk of the electrolyte (Cinh) to the degree of surface coverage (θ) as Equation:
(15)
where Cinh is cefixime concentration and Kads is the equilibrium constant of the adsorption process.
In order to evaluate the strength of the interactions between the inhibitor molecules and the metal surface, the values of adsorption equilibrium constant
Kads were computed using the intercepts of the straight lines on
-axis. The
calculated adsorption equilibrium constant was related to the standard free energy of adsorption by the following equation [37]:
(16)
![]()
Figure 4. Langmuir adsorption isotherm for cefixime on copper surface in 1M HNO3.
![]()
Table 1. Regression parameters of Langmuir isotherm.
In the above equation [38], 55.5 is the concentration of water in mol·L−1, T is the absolute temperature while R is the universal gas constant. The values of
and the other adsorption thermodynamic functions are gathered in Table 2.
The negative values of
indicate that the adsorption process is spontaneous and the adsorbed layer on the aluminum surface is stable [9]. Generally, [39] [40], values of −20 kJ·mol−1 or less negative are consistent with the electrostatic interactions between the charged metal and the inhibitor i.e. physisorption. The values around −40 kJ·mol−1 or more negative are associated with chemisorption, as a result of sharing or transfer of unshared electron pair or π-electrons of organic molecules to the metal surface to form a coordinate type of bond. In the present work,
change ranges from –38.00 to –36.14 kJ·mol−1 indicating both physisorption and chemisorption. The standard adsorption enthalpy change
and the standard adsorption entropy change
are correlated with standard Gibbs free energy through the relation:
(17)
and
are obtained respectively as the intercept and the negative of the slope of the straight line obtained by plotting
versus T (Figure 5). The change in adsorption enthalpy
is negative, showing an exothermic process. The literature [41] stated that an exothermic process means either physisorption
![]()
Table 2. Adsorption thermodynamic functions.
![]()
Figure 5.
versus T for the adsorption of cefixime on copper in 1M HNO3.
or chemisorption. Therefore, this result confirms that the process of adsorption is both physisorption and chemisorption. The change in standard adsorption entropy
is positive, meaning that disorder increases during the adsorption process. This situation can be attributed to desorption of water molecules replaced by the inhibitor.
3.1.3. Effect of the Temperature and Activation Parameters
The activation energy (
) can be obtained by using Arrhenius equation (Equation (18)):
(18)
where CR is the corrosion rate and A is the Arrhenius pre-exponential constant. A plot of
versus
yields a straight line (Figure 6) with
as slope and
as intercept.
The other activation parameters for the corrosion process were calculated from the Arrhenius equation:
(19)
![]()
Figure 6. Arrhenius plots for copper corrosion in 1M HNO3 without and with different concentrations of cefixime.
where
is the change in activation entropy,
is the change in activation enthalpy and
is the Avogadro number and h is the Planck’s constant.
Figure 7 gives the plot of
versus
.
The slope
and the intercept
of each
straight-line lead to the values of activation enthalpy change and activation entropy change (Table 3).
From Table 3, it seems that
and
varied in the same manner increasing with the concentration, probably due to the thermodynamic relation between them (
). It can be seen that the values of
are higher in the inhibited solutions than those in uninhibited solutions. On the other hand, the higher values of
in the presence of inhibitor compared to that in its absence and the decrease of the inhibition efficiency (IE) with the increase in temperature can be interpreted as an indication of predominant physisorption process [4] [42] [43]. Moreover, the positive signs of
pointed out the endothermic effect of the copper dissolution process. The value of
is higher for the inhibited solution than that for the uninhibited solution. This phenomenon suggested that a randomness decrease occurred from reactants to the activated complex. This might be the result of the adsorption of organic inhibitor molecules from the acidic solution which could be seen as a quasi-substitution process between the inhibitor in the aqueous phase and water molecules at the metal surface [44].
3.2. Quantum Chemistry Study
3.2.1. Global Reactivity
The values of selected quantum chemical parameters computed for the studied cefixime molecule by using DFT methods are listed in Table 4.
According to the frontier molecular orbital (FMO) theory, of chemical reactivity,
![]()
Figure 7. Transition state plots for copper corrosion in 1M HNO3 with or without different concentrations of cefixime.
![]()
Table 3. Calculated activation energy, activation enthalpy change and activation entropy change.
![]()
Table 4. Molecular and global reactivity descriptors of cefixime.
transition of electron is due to interaction between highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of reacting species [45] [46]. These HOMO and LUMO energies [47] are very important in defining the reactivity of a given molecule. EHOMO [48] is often associated with the electron donating ability of a molecule. High EHOMO values indicate that the molecule has a tendency to donate electrons to appropriate acceptor molecules with low energy empty molecular orbital. This high value indicates the tendency to donate electrons to empty molecular orbital of copper ions (Cu2+: [Ar]3d9). Increasing values of the EHOMO facilitate adsorption (and therefore inhibition) by influencing the transport process through the adsorbed layer [49]. On the other hand, the LUMO energy [48], indicates the ability of the molecule to accept electrons. The lower the value of EHOMO, the more probable it is that the molecule accepts electrons. In this work, the higher value of EHOMO (−5.8680 eV) and the lower value of ELUMO (−2.0510 eV) could involve charge sharing or charge transfer from the inhibiting molecule to the metal surface [50]. That could explain good inhibition efficiency of cefixime. In the same way, low values of the energy gap (ΔΕ = ΕLUMO − ΕHOMO) lead to high inhibition efficiencies because [36] the energy to remove an electron from the last occupied orbital will be low. Literature [51] revealed that excellent corrosion inhibitors are organic compounds which not only offer electrons to unoccupied orbital of metal but also accept free electrons from the metal. A molecule with a low energy gap [36] is more polarizable and is generally associated with high chemical reactivity and is considered a soft molecule. The value of ΔE(3.8170 eV) is low compared with that of many molecules in the literature, suggesting good inhibition efficiency.
Another important electronic parameter calculated is the dipole moment (μ) that results from non-uniform distribution of charges on atoms in the molecule. Several authors point out that low values of dipole moment [52] promote accumulation of the inhibitor molecules in the surface layer and therefore higher inhibition efficiency. However, many papers indicate that inhibition efficiency increases with rising values of dipole moment. On the other hand, the survey of the literature [53] [54], reveals that several irregularities were appeared in the case of correlation of dipole moment with inhibitor efficiency. So, in general [55], there is no significant relationship between dipole moment values and inhibition efficiencies.
Absolute hardness and softness are important parameters to measure the molecular stability and reactivity of a molecule. The chemical hardness fundamentally represents the resistance towards the deformation or polarization of the electron cloud of atoms, ions or molecules under small perturbation of chemical reaction. A hard molecule has a large energy gap and a soft molecule has a small energy gap [56]. In the present paper, the studied molecule has a low hardness value (1.9085 eV) and a high value of softness (0.5240 (eV)−1) when compared [57] [58] with molecules in the literature.
The ionization potential (I) and the electronic affinity (A) are respectively (5.8680 eV) and (2.0510 eV). This low value of (I) and the high value of electron affinity indicate the capacity of the molecule both to donate and accept electron. The electronegativity (χ) indicates the capacity of a system to attract electrons. In our work the low value of the electronegativity of the studied molecule (
) when compared to that of copper (
) shows that copper has the better attraction capacity. Then the low value of hardness (1.9085 eV) confirms the relatively higher value of the fraction of electrons transferred (ΔN = 0.0840) indicating a possible motion of electrons from the inhibitor to the metal.
The electrophilicity index measures the propensity of chemical species to accept electrons; a high value of electrophilicity index describes a good electrophile while a small value of electrophilicity index describes a good nucleophile. In this work the obtained value (ω = 4.1073 eV) shows the good capacity of cefixime to accept electrons. The HOMO and LUMO diagrams of the studied drug are presented in Figure 8.
As seen in Figure 8, the density HOMO is distributed around the five-membered ring containing heteroatoms whereas, the LUMO density is distributed almost homogeneously throughout the molecule. So, these regions are probably the most active areas where transfer of electrons could be achieved (from the inhibitor molecule to copper or vice-versa).
3.2.2. Local Reactivity
Local reactivity descriptors including atomic charges, condensed Fukui functionsand dual descriptor are collected in Table 5.
It can be clearly observed from shaded rows in Table 5 that (15C) with the maximum value of
and positive value of
is the most probable nucleophilic attack site, while (35C) with the maximum value of
and negative value of
is the most probable electrophilic attack site.
![]()
Figure 8. HOMO and LUMO diagrams of cefixime by B3LYP/6-31G (d, p).
![]()
![]()
Table 5. Calculated Mulliken atomic charges, Fukui functions and dual descriptor by DFT B3YLP 6-31/G (d, p).
4. Conclusion
Cefixime drug shows good inhibition properties for copper corrosion in 1M HNO3. The inhibition efficiency increases with increasing concentration in the studied inhibitor but decreases with a rise in temperature. Adsorption of cefixime follows the Langmuir adsorption isotherm. The adsorption and activation thermodynamic functions reveal a spontaneous adsorption process of the studied molecule onto copper and both physisorption and chemisorption mechanisms with the predominance of physisorption. Quantum chemical calculations data from B3LYP/6-31G (d, p) fit well the experimental results.