Synthesis, Characterization, and Antioxidation Evaluation of Novel Spiro-5-(fluoren-9’-yl)-6-azauracil and Their N,N-Dialkyl Derivatives ()
1. Introduction
6-Azauracil (1,2,4-triazine-3,5(2H,4H)-dione) A is one of the important families of heterocyclic nitrogen systems as biodynamics targets. Recently much effort has been exerted on the synthesis, chemical reactiveness, physical properties, and biological evaluation of 6-azauracil A and their N,N-Disubstituted derivatives. The results have shown that these group of compounds exhibit anticancer [1], antiviral [2] [3], antimalarial [4], herbicidal [5] and antimicrobial [6] [7] [8] [9] [10] behavior, while the N4-fluoro aromatic substituted uracils B possess anticancer [11], anti-depressant hypnotic [12], antiallergic, anxiolytic, antidepressant [13] and anticoccidial properties (Figure 1). JUSTEA patent report “Certain 6-azauracils and derivatives thereof are known in the art. U.S. Pat. Nos. 3,905,971
![]()
Figure 1. Important medicinal 6-azauracils.
and 3,912,723 disclose certain 2-phenyl-as-triazine-3,5(2H,4H)diones and certain 2-substituted-phenyl-as-triazine-3,5(2H,4H)diones and their use as agents for the control of coccidiosis. U.S. Pat. Nos. 3,883,527 and 3,883,528 disclose processes for producing certain 2-aryl-as-triazine-3,5(2H,4H)-diones, which are useful as coccidiostats”.
Accordingly, the present work focused on investigation for novel N-flurinyl-9-spiro-6-azauracils, in view of their antioxidant properties.
2. Chemistry
This investigation aims to synthesize novel 6-azauracils derivatives as antioxidant probs. Synthesis strategy starts with the synthesis of spiro 5-(fluoren-9’-yl)-6-azauracil 3, followed by the formation of N,N-disubstituted 6-azauracil 4 - 17, via a simple alkylation. Thus, condensation of fluoren-9-one with semicarbazide, HCl in reflux with MeOH for 1 h to yeilde the semicarbizone 1, which upon reaction of HCN under reflux yeilds N-(carbonitryl)-N-(flurin-9’-yl)-semicarbized 2. Acidic hydrolysis of compound 2 achieved the target spiro 5-(flurin-9’-yl)-hedxahydro-6-azauracil 3 (Scheme 1). Compound 3 is called the flurinyl spiro-6-azauracile.
The N1,N2-disubstituted 6-azauracils 3 are used as potential inhibitors [14]. Thus, N-Methylation of compound 3 by MeI/1% aq. KOH at room temperature gives 1,3-dimethyl-Spiro 5-(fluoren-9-yl)-6-azauracil 4, while hydroxymethylation of 3 by refluxing with MeOH/HCHO, yielded 1,3-dihydroxymethyl-spiro-5-(fluoren-9’-yl)-6-azauracil 5. Similarly, Mannich bases 6 and 7 obtained by the by the refluxing of compound compound 3 with secondary amines (such as piperidine and/or morpholine) in MeOH/HCHO (Scheme 2).
Moreover, reflux compound 3 with primary aromatic amines for example; sulfanilamide 4-amine-3-hydroxy-naphthine sulfonic acid and 4-amino antipyrine drug give the 1,3-di(substituted amino) methyl-spiro-5-(fluoren-9’-yl)-6-azauracils 8 - 10 (Scheme 3).
In general, the introduction of acetamide urea and thiourea to heterocyclic moiety almost improves their properties. Thus, reflux compound 3 with acetamide, N-phenyl urea and N-phenyl thiourea produced 1,3-di(acetyl-spiro-5-(flurin-9’-yl)-6-azauracil 11, 1,3-Dianilido-spiro-5-(flurin-9-yl)-6-azauracil 12 and 1,3-dithianilido-spiro-5-(flurin-9’-yl)-6-azauracil 13 respectively (Scheme 4).
Furthermore, the room temperature reaction stirring of compound 3 with formyl acetate (CH3COOCHO) in ether for 4 - 6 h, furnished 1,3-diformyl-spiro-5-(flurin-9-yl)-6-azauracil 14 (Scheme 5). Reactivity of compound 4 primarily evaluated by reflux with primary amine for example aniline and hydrazine to give the imino 15 and hydrazone 16 derivatives.
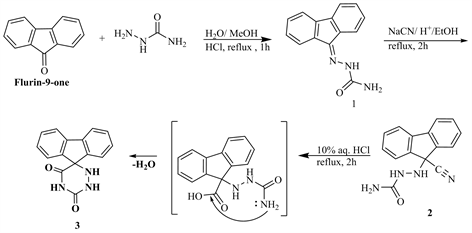
Scheme 1. Synthesis of flurinyl spiro-6-azauracile 3.
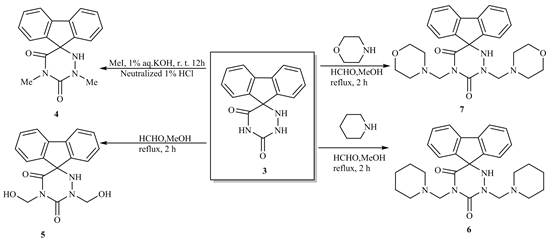
Scheme 2. Formation of compounds 4 - 7.
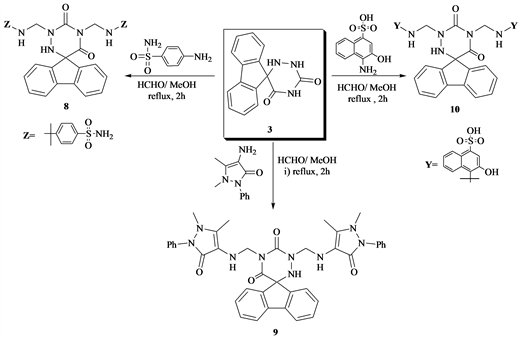
Scheme 3. Formation of compounds 8 - 10 from 3.
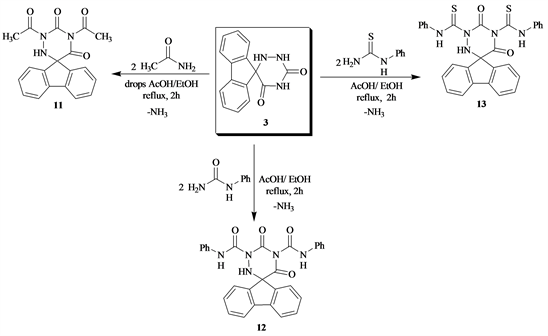
Scheme 4. Formation of compound 11 - 13 from 3.
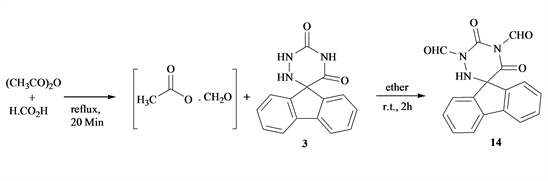
Scheme 5. Formation of compound 14 from 3 and formyl acetate.
Fusion heating of compound 16, furnished the thermal cyclisation compound 1,2,4-triazolo [1,2,4]trizino-1,2,4-trizole 17. Likewise, Compound 17 obtained from cyclocondensation reaction of 14 with hydrazine hydrate (Scheme 6).
Moreover, condensation of compound 14 with thiosemicarbazide in acetic acid afforded the thiosemicarbazone 18 which upon ring closure reactions with malonic acid in refluxing AcOH, yielded the thiobarbituric acid derivatives 19 (Scheme 7).
3. Results and Discussion
6-Azauracil derivatives showed significant biological effects in comparison with uracil moiety, 6-azauracil enhanced the electronegativities over the structures center. This improves distribution, dielectric constant and hydrophobic properties within the body [1] [2]. Correspondingly, 6-azauracil used as amphipathic prodrugs of 1,2-diol drugs via the regioselective enzymatic protocol [3]. Thus, this investigation tends to synthesis a novel 6-azauracil followed by obtaining their 1,3-disubstinted spiro-(5-fluoren-9’-yl)-6-azauracil 4 - 19 (Schemes 1-7). Structures of the new targets are deduced from their elemental analysis and spectral measurement.
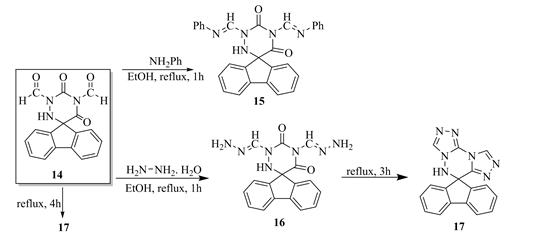
Scheme 6. Formation of compound 15 - 17 from 14.
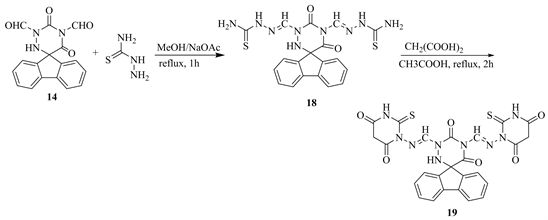
Scheme 7. Formation of 19, 18 from 14.
IR spectrum of compound 3 showed ν−1 at 3200 - 3100 cm−1 for 3 NH of 1,3- and 6-position with ν−1 at 1700 - 1680 cm−1 for two C=O. While, all IR spectra of compounds 4 - 19 recorded the lack of N1 and N3 of 6-azauracil which confirm that substitution reactions tack place on NH, with appearances of two C=O at ν−1 1710 - 1680 cm−1 for 2- and 4-positions. IR spectrum of 17 showed a lack of both 1NH and 3NH and C=O of 6-azauricle, while that of 18 recorded ν−1 3200 - 3100, 1200 attribute to both C=S and -CH2- groups respectively which confirm that full fused heteropoly cyclic System 17.
The 1H NMR spectrum, showed broad beak at 14 - 11 ppm attribute to presence the unreactive NH at 6-position of 6-azauracil.
Only, compounds 4 - 11 showed at ~4 ppm attribute to CH₂-N for N1and N3, while compounds 8, 9, 10, 12, 13 and 15 recorded the additional, new aromatic protons at 8 - 6 ppm, while that of 5 showed 5.5 ppm for OH protons.
In addition, the oriental aromatic protons of flurin-9’-yl moiety shows aromatic multiple at 6 - 8 ppm for compounds 3 - 18. Compound 14 exhibit peak at 9.5 ppm for the aldehydic proton, which lack’s in the structure of 15 - 18. 13C NMR spectral study of the new synthesized compound 4 - 11 showed at 30-15 ppm attribute to present of aliphatic carbon. Only the compound 13, 18 and 19 recorded at 180 - 170 ppm for C = S carbons. All the compounds, showed at 130 - 120 ppm for the aromatic carbon with at 170 - 160 ppm for C = O carbons which disappear in the structure of 17. Only compound 19 recorded signals at 40 ppm attribute a cyclic CH2 of thiobarbituric acids.
Mass fragmentation pattern shows the molecular ion peaks and base peak. This observation explains the degree of stabilities for the study systems, non-aromatic, thus, the base peak of the compound 3, 6 and 7 is m/z 152 for C12H9 while that of compounds of 12, 13 and 17 is 56.63 attribute to a type of cyclic (Table 1). While Figure 2 and Figure 3 showed two examples for suggested mass cyclic fragmentation of compound 3 and 17.
![]()
![]()
Table 1. Mass fragmentation study of new compounds 3 - 17 (M/Z/Int. %).
![]()
Figure 2. Mass fragmentation pattren of 3.
![]()
Figure 3. Mass fragmentation pattern of compound 17.
4. Experimental
The commercial chemicals and solvents used in the synthesis were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO), Fisher Scientific Inc. (Springfield, NJ), or Lancaster (Windham, NH) and were used without further purification. Analytical grade reagents were purchased from standard commercial sources. Melting points determined with an electrothermal Bibby Stuart Scientific melting point sample (UK). A Perkin Elmer Model RXI-FT-IR system 55529 was used for recording the IR spectra of the prepared compounds. A Bruker advance DPX 400 MHz model uses TMS as internal standard was used for recording the 1H and 13C NMR spectra of the compounds on deuterated DMSO-D6. A GC-MS-GP 1000 Ex model was used for recording the mass spectra of the compounds. Electronic spectra were recorded in ethanol on Shimadzu UV and visible 310 IPC Spectrophotometer. Elemental analysis was performed in micro analytical center of Cairo University, Cairo, Egypt.
1) Semicarbazon-N-(fluoren-9’-yl) (1)
Flurin-9-one (0.01 mol) and semicarbazide. HCl (0.01 mol, in 10 ml H2O) in MeOH (20 ml) refluxed for 1 h, cooled then poured onto ice. The solid obtained filtered and crystallized from MeOH to give 1. Yield 80%. m.p. 210˚C - 212˚C. IR (νcm−1): 3362, 3200, 3127 (NH∙NH2) 1666 (CONH), 1626 (NH2), 1571 (C=N), 1317 (NCN), 949, 916, 877 (aromatic ring). 1HNMR (DMSO-d6) (δ): 10.71 (s, NH), 8.51 (d, 4H, aromatic), 7.1 - 7.6 (m, 2H, aromatic), 7.19 - 7.15, 7.07 - 6.76 (m, 2H, aromatic), 3.35 (s, 2H, NH2). 13CNMR (DMSO-d6) (δ): 162 (C=O), 140 (C=N), 120 - 116 (aromatic carbons), 109 (C-C). Anal.%Calcd for C14H11N3O (237.26): C, 70.87; H, 4.67; N, 17.71. Found: C, 70.66; H, 4.51; N, 17.49.
2) Fluoren-9-(N-semicarbazide)-9-carbonitrile (2)
A mixture of 1 (0.01 mol) and NaCN (0.01 mol, in 10 ml H2O) in EtOH/AcOH (1:1, 50 ml) refluxed 2 h, cooled then poured onto ice. The solid product filtered off and crystallized from EtOH to give 2, yield 70%, m.p. 230˚C - 232˚C. IR (νcm−1): 3203, 3128 (NH, NH), 2220 (CN), 1669 (C=O), 1573 (C=N), 1356 (NCN), 1170 (N-N), 946, 916, 870 (aromatic rings). 1HNMR (DMSO-d6) (δ): 8.31 (s, NH), 8.61 - 8.83 (m, 2H, aromatic), 7.1 - 7.6 (m, 1H, aromatic), 7.21 - 7.43, 7.07 - 6.76 (m, 2H, aromatic), 6.35 (s, 2H, NH2). 13CNMR (DMSO-d6) (δ): 163.7 (C=O), 133.8 (C=N), 120.12 - 115.66 (aromatic carbons). Anal.%Calcd for C15H12N4O (264.29): C, 68.17; H, 4.58; N, 21.20. Found: C, 68.11; H, 4.39; N, 20.09.
3) Spiro-(5-fluoren-9’-yl)-6-azauracil (3)
Compound 2 (1 gm, 0.01 mol) and aq. HCl (10%, 10 ml) heated under reflux 2 h, cooled, the resultant solid, filtered off and crystallized from MeOH to give 3; Yield 66%; m.p. 250˚C - 252˚C, IR (νcm−1): 3459 (OH), 1668 (C=O), 1617 (C=C), 1596 (C=N), 1316 (NCN), 948, 876 (aromatic rings). 1HNMR (DMSO-d6) (δ): 11.5 - 12.11 (m, 3NH), 8.55 (m, 4H, aromatic), 7.12 (d, 4H, aromatic). 13CNMR (DMSO-d6) (δ): 169.6 (C=O), 141.12 (C=O), 130.99 (C=N), 120.76 - 117.11 (aromatic carbons), 89.7 (C-C-N). Anal.%Calcd for C15H11N3O2 (237.26): C, 67.92; H, 4.18; N, 15.84. Found: C, 67.79; H, 4.70; N, 15.80.
4) 1,3-Dimethyl-spiro-5-(fluoren-9’-yl)-6-azauracil (4)
A mixture of 3 (0.01 mol), MeI (0.01 mol) in aq. KOH (1%, 50 ml) stirred for 12 h at room temperature. The reaction neutralized by dil. HCl treated with MeOH. The yielded solid filtered off and crystallized from dioxan to give 4. Yield 60% m.p. 150˚C - 152˚C IR (νcm−1): 3380 (OH), 3320 (NH), 3080 (aromatic CH), 2950, 2870 (aliphatic CH), 1700, 1680 (2C=O), 1490, 1440 (deformation CH2), 1310 (NCN), 940, 910 (aromatic rings). 1HNMR (DMSO-d6) (δ): 7.90 (m, 4H, aromatic), 7.28 (m, 4H, aromatic protons), 3.51 (s, 3H, CH3), 3.2 (s, 3H, CH3). 13CNMR (DMSO-d6) (δ): 162.2, 159.4 (2C=O), 130.81 - 121.72 (aromatic carbons), 112.89 - 110.70 (C-C). Anal.%Calcd for C17H15N3O2 (293.33): C, 69.61; H, 5.15; N, 14.33. Found: C, 69.51; H, 5.05; N, 14.12.
5) 1,3-Dihydroxymethyl-spiro-5-(fluoren-9’-yl)-6-azauracil (5)
A mixture of 3 (0.01 mol), HCHO (0.02 mol) in MeOH (50 ml) refluxed 2 h then poured onto ice. The solid produced filtered off and Crystallized from MeOH to give 5. Yield 60%, m.p. 160˚C - 162˚C. IR (νcm−1): 3380 (NH2), 3050 (aromatic CH), 2980, 2890 (aliphatic CH), 1710, 1680 (2C=O), 1560 (C=N), 1490, 1440 (deformation CH3), 1310 (NCN), 960, 910 (aromatic rings). 1HNMR (DMSO-d6) (δ): 8.87 (m, 4H, aromatic), 7.93 (m, 4H, aromatic protons), 5.9 (s, 1H, OH), 5.32 (s, 2H, CH2), 5.22 (s, 2H, CH2). 13CNMR (DMSO-d6) (δ): 142.2, 155.4 (2C=O), 141.81 - 126.72 (aromatic carbons), 77.9 (CH2), 68.6 (CH2). Anal.%Calcd for C17H15N3O4 (325.32): C, 62.76; H, 4.65; N, 12.92. Found: C, 62.55; H, 4.39; N, 12.80.
6) Mannich Bases 6 & 7
A mixture of 3 (0.01 mol) and sec. amines as piperidine and/or morpholine (0.02 mol), HCHO (0.02 mol) and MeOH (50 ml) refluxed 2 h, cooled then poured onto ice. The solid obtained filtered off and crystallized from MeOH to give 6 and/or 7 respectively.
6, yield 70%, m.p. 195˚C - 196˚C.
7, yield 72%, m.p. 200˚C - 201˚C.
6, Anal.%Calcd for C27H33N5O2 (459.59): C, 70.56; H, 7.24; N, 15.24. Found: C, 70.44; H, 7.12; N, 15.10.
7, Anal.%Calcd for C25H29N5O2 (459.59): C, 64.78; H, 6.31; N, 15.11; O, 13.81 Found: C, 64.58; H, 6.21; N, 15.31; O, 13.41.
6, IR (νcm−1): 3059 (NH), 2933 (CH2), 1712, 1665 (2C=O), 1610 (C=C), 1598 (C=N), 1471, 1448 (deformation CH2), 1179 (NCN), 949, 917 (aromatic rings).
7, IR (νcm−1): 3310 (NH), 3050 (aromatic CH) 2980 (aliphatic CH), 1680, 1644 (2C=O), 1578 (C=N), 1478, 1449 (deformation CH2), 1303 (NCN), 1681 (C-O-C), 950, 915 (aromatic rings).
6, 1HNMR (DMSO-d6) (δ): 7.5 - 8.0 (m, 4H, aromatic protons), 8.1 - 8.4 (m, 4H, aromatic protons), 1.30 (pent, 4H, 2CH2), 1.45 - 1.50 (m, 2H, CH2), 2.4 (t, 8H, 4CH2), 5.1 (s, 1H, CH2-N), 5.1 (s, 1H, NH).
7, 1HNMR (DMSO-d6) (δ): 7.30 - 7.90 (m, 4H, aromatic protons), 8.0 - 8.2 (m, 4H, aromatic protons), 1.30 (pent, 4H, 2CH2), 1.45 - 1.50 (m, 4H, 2CH2), 5.1 (s, 1H, CH2-N), 5.1 (s, 1H, NH). 13CNMR (DMSO-d6) (δ): 148.2, 156.4 (2C=O), 140.66 - 125.12 (aromatic carbons), 84.9(CH2), 77.9, 65.1 (2CH2-N), 66.4, 52.8 (4 CH2).
7) 1,3-Di(4’-tollylsulfonamido) methyl-spiro-(5-fluoren-9-yl)-6-azauracile (8)
A mixture of 3 (0.01 mol) and sulfanilamide drug (0.02 mol) with MeOH (50 ml), HCHO (0.02 mol) refluxed 2 h, cooled then poured onto ice. The solid yielded filtered off and crystallized from EtOH to give 8. Yield 66% m.p. 180˚C - 181˚C. IR (νcm−1): 3461, 3368, 3212 (NH, NHCH2, NH2, SO2), 1702, 1669 (2C=O), 1619 (C=C), 1595 (C=N), 1485, 1459 (deformation CH2), 1304 (SO2NH), 1177 (C-N), 946, 916, 824 (aromatic rings). 1HNMR (DMSO-d6) (δ): 7.90 - 7.85 (m, 4H aromatic protons), 7.71 - 7.52 (m, 4H, aromatic protons), 7.54 - 7.12 (m, 8H, phenyl protons), 6.89 (s, 4H, 2NH2), 6.3 (s, 1H, NH), 6.2(s, 1H, NH), 5.55 (s,1H, NH), 4.9, 4.52 (s, 4H, 2CH2). 13CNMR (DMSO-d6) (δ): 162.2, 156.4 (2C=O), 140.66 - 125.12 (aromatic carbons), 84.9 (CH2), 77.9, 65.1 (2CH2-N), 66.4, 52.8 (4CH2). Anal.%Calcd for C29H27N7O6S2 (633.70): C, 56.34; H, 3.68; N, 12.17; O, 16.68; S, 11.14. Found: C, 56.24; H, 3.78; N, 12.57; O, 16.68; S, 11.69.
8) 1,3-Di(1’-phenyl-2’,3’-dimethyl-5’-exo-pyrazol-4-yl)aminomethyl-spiro-(5’-fluoren-9-yl)-6-azauracil (9)
A mixture of 3 (0.01 mol) and 4-aminoantipyrine (0.02 mol), HCHO, (0.02 mol), MeOH (50 ml) refluxed 2 h, cooled, the solid obtained filtered off and crystallized from EtOH to give 9. Yield 60%; m.p. 205˚C - 207˚C. IR (νcm−1): 3400 (NH), 3302, 3131 (NH, NH), 3050 (aromatic CH), 1702, 1670 (C=O), 1618 (C=C), 1595, 1574 (C=N), 1485, 422 (deformation CH3, CH2), 946, 917, 877 (aromatic rings). Anal.%Calcd for C39H37N9O4 (695.78): C, 67.32; H, 5.36; N, 18.12; O, 9.20 Found: C, 67.11; H, 5.18; N, 18.02; O, 9.00
9) 1,3-Di(3’-hydroxy-naphthalin-1’-sulfonic acid-4’-amino)methyl-spiro-5-(fluoren-9-yl)-6-azauracil (10)
A mixture of 3 (0.01 mol), 3-amino-3-hydroxy-naphthaline-sulfonic acid (0.02 mol), HCHO (0.02 mol), MeOH (50 ml) refluxed 2 h, cooled. The yielded solid, filtered off and crystallized from MeOH to give 10, yield 59%; m.p.289˚C - 290˚C, IR (νcm−1): 3550 (OH), 3380, 3310, 3098 (NH), 2885 (aliphatic CH), 2640 (OH), 1685, 1655 (C=O), 1619 (C=C), 1576 (C=N), 1469, 1433 (deformation CH2), 1182 (SO2), 980, 939, 893, 860 (aromatic rings). 1HNMR (DMSO-d6) (δ): 8.30 - 8.11 (m, 4H aromatic protons), 7.91 - 7.72 (m, 4H, aromatic protons), 7.54 - 7.12 (m, 8H, phenyl protons), 6.89 (s, 4H, 2NH2), 6.3 (s, 1H, NH), 6.2 (s, 1H, NH), 5.55 (s, 1H, NH), 4.9, 4.52 (s, 4H, 2CH2). 13CNMR (DMSO-d6) (δ): 162.2, 156.4 (2C=O), 140.66 - 125.12 (aromatic carbons), 84.9 (CH2), 77.9, 65.1 (2CH2-N), 66.4, 52.8 (4CH2). Anal.%Calcd for C37H29N3O10S2 (767.78): C, 57.88; H, 3.81; N, 9.12; O, 20.84; S, 8.35. Found: C, 57.59; H, 3.91; N, 9.02; O, 20.66; S, 8.15.
10) 1,3-Di(acetamido)-spiro-5-(fluoren-9-yl)-6-azauracil (11)
A mixture of 3 (0.01 mol) and acetamide (0.02 mol), in AcOH/EtOH (10 ml, 1:1) refluxed 2 h, cooled then poured onto ice. The produced solid, filtered off and crystallized from EtOH to give 11, yield 68%; m.p. 233˚C - 235˚C. IR (νcm−1): 3363 (NH), 3200 (NH), 1710, 1680, 1668 (C=O), 1596 (C=N), 1571 (C=N), 1486, 1459 (deformation CH3), 1360 (NCON), 946, 918, 878, 781 (aromatic rings). 1HNMR (DMSO-d6) (δ): 9.30 - 9.11 (m, 4H aromatic protons), 8.93 - 8.82 (m, 4H, aromatic protons), 5.0 (s, 1H, NH), 2.40 (s, 3H, CH3), 2.22 (s, 3H, CH3). 13CNMR (DMSO-d6) (δ): 175.2, 172.4 (2C=O), 152.8 (2C=O), 141.66 - 134.12 (aromatic carbons), 85.9 (spiro C), 25.3, 20.5 (2CH3). Anal.%Calcd for C19H15N3O4 (439.35): C, 65.32; H, 4.33; N, 12.03; O, 18.32. Found: C, 65.92; H, 4.83; N, 12.93; O, 18.02.
11) 1,3-Di(Anilido)-spiro-(5-fluoren-9-yl)-6-aza-uracil (12)
A mixture of 3 (0.01 mol), N-phenylurea (0.02 mol) in AcOH/EtOH (1:1, 10 ml) refluxed for 2 h, cooled, then poured onto ice. The solid obtained filtered off and crystallized from MeOH to give 12, yield 70% m.p. 225˚C - 227˚C. IR (νcm−1): 3300, 3202, 3128 (NH), 3060 (aromatic CH), 1680, 1668, 1630 (C=O, CONH), 1615 (C=C), 1595 (C=N), 1317 (NCON), 1176 (C-N), 946, 917, 880, 781 (aromatic rings). 1HNMR (DMSO-d6) (δ): 9.22 - 9.54 (m, 4H aromatic protons), 8.93 - 8.82 (m, 4H, aromatic protons), 7.54 - 7.12 (m, 8H, phenyl protons), 5.5 (s, 1H, NH). 13CNMR (DMSO-d6) (δ): 177.9, 172.33 (2C=O), 152.8 (2C=O), 144.66 - 134.12 (aromatic carbons), 88.1 (spiro C). Anal.%Calcd for C29H21N5O4 (503.52): C, 69.18; H, 4.20; N, 13.91; O, 12.71. Found: C, 69.00; H, 4.77; N, 13.99; O, 12.01.
12) 1,3-Di(thiaanilido)-spiro-(5-fluoren-9’-yl)-6-aza-uracil (13)
A mixture of 3 (0.01 mol), N-phenylthiourea (0.02 mol), in HCHO (0.02 mol) in MeOH (50 ml) refluxed for 2 h, cooled, then poured onto ice. The solid produced filtered off and crystallized from EtOH to give 13, yield 71% m.p. 235˚C - 237˚C. IR (νcm−1): 3310, 3205, 3057 (NH), 1712, 1669 (C=O), 1610 (C=C), 1597 (C=N), 1357 (NCSN), 1171 (C-S), 946, 917, 877, 812, 782 (aromatic rings). 1HNMR (DMSO-d6) (δ): 8.90 (s, 2H, 2NH), 8.56 - 8.11 (m, 4H aromatic protons), 7.94 - 7.55 (m, 4H, aromatic protons), 5.0 (s, 1H, NH), 2.40 (s, 3H, CH3), 2.22 (s, 3H, CH3). 13CNMR (DMSO-d6) (δ): 175.2, 172.4 (2C=O), 152.8 (2C=O), 141.66 - 134.12 (aromatic carbons), 85.9 (spiro C), 25.3, 20.5 (2CH3). Anal.%Calcd for C29H21N5O2S2 (535.64): C, 65.03; H, 3.95; N, 13.08; O, 5.97; S, 11.97. Found: C, 64.89; H, 3.77; N, 12.79; O, 5.97; S, 11.89.
13) 1,3-Diformyl-spiro-(5-fluoren-9’-yl)-6-aza-uracil (14)
To compound 3 (0.01 mol), in dry ethylether, 100 ml; formylacetate (0.02 mol) added with stirring at room temperature for 12 h, The solid obtained filtered off and crystallized from dioxan to give 14. [Ac2O + HCOOH (1:1) were refluxed 20 min then cooled to give formyl acetate]. Compound 14, yield 80%; m.p. 280˚C - 282˚C, IR (νcm−1): 3100 (NH), 1710, 1680 (C=O), 920, 860, 810 (aromatic rings). 1HNMR (DMSO-d6) (δ): 8.21 (s, 1H, CH), 8.05 - 7.95 (m, 4H aromatic protons), 7.82 - 7.55 (m, 4H, aromatic protons), 5.9 (s, 1H, NH). 13CNMR (DMSO-d6) (δ): 168.7, 152.4 (2C=O), 161.8 (C=O), 144.66 - 124.12 (aromatic carbons), 88.1 (spiro C). Anal.%Calcd for C17H11N3O4 (321.29): C, 63.55; H, 3.45; N, 13.08; O, 19.92. Found: C, 63.40; H, 3.15; N, 12.89; O, 19.00.
14) 1,3-Dischiff base-spiro-(5-fluoren-9’-yl)-6-aza-uracil (15)
A mixture of 14 (0.01 mol) and anhydrous aniline (0.02 mol) in abs. EtOH (20 ml) warmed for 1 h, cooled. The solid produced filtered off and crystallized from EtOH to give 15, yield 75%; m.p. 150 - 152. IR (νcm−1): 3222(NH), 1709, 1660 (2C=O) 1670 (C=C), 1190 (P=O), 814 (substituted phenyl). 1HNMR (DMSO-d6) (δ): 8.91 (s, 1H, CH), 7.95 (d, 2H aromatic protons), 7.54 (d, 2H aromatic protons), 7.32 - 7.25 (m, 6H, aromatic protons), 6.65 (d, 2H aromatic protons) 5.34 (s, 2H, NH2), 5.09 (s, 1H, NH). Anal.%Calcd for C29H21N5O2 (471.5): C, 73.87; H, 4.49; N, 14.85; O, 6.79. Found: C, 73.07; H, 4.79; N, 14.55; O, 6.69.
15) 1,3-Di(hydrazono)-spiro-(5-fluoren-9’-yl)-6-aza-uracil (16)
A mixture of 14 (0.01 mol) and hydrazine hydrate (100%. 0.022 mol) in abs. EtOH (100 ml) warmed under reflux for 1 h, cooled. The resultant solid filtered off and crystallized from EtOH to give 16, yield 60%; m.p. 100˚C - 102˚C. IR (νcm−1): 3202 (NH), 1719, 1630 (2C=O) 1690 (C=C), 1208 (C=N), 930, 850, 830 (aromatic rings). Anal.%Calcd for C17H15N7O2 (349.35): C, 58.45; H, 4.33; N, 28.07; O, 9.16. Found: C, 58.05; H, 4.90; N, 29.07; O, 9.81.
16) 5H-Spiro-6-(5-fluoren-9’-yl)-1,2,4-Triazizolo [3,4-b]-1,2,4-trizolo [5,4-d] [1,2,4]triazene (17)
a) A mixture of 14 (0.01 mol) and hydrazine hydrate (0.022 mol) in abs.EtOH (100 ml) refluxed for 4 h, cooled. The solid obtained filtered off and crystalized from THF to give 17, yield 70%; m.p. > 300.
b) Compound 16 was heated above its melting point and mixed melting point not change.
Anal.%Calcd for C17H11N7 (313.31): C, 65.17; H, 3.54; N, 31.29 Found: C, 65.98; H, 3.45; N, 31.02.
5. The Anti-Oxidant Evaluation
Antioxidants are substances that can prevent or slow damage to cells caused by unstable free radicals [15] [16] [17]. Also, the molecules that can neutralize free radicals by accepting or donating electrons to eliminate the unpaired condition of the radicals. Oxidation reactions can produce a free radical, which starts the chain reactions that damage cells. The oxidation damage to DNA, proteins, and other macromolecules, have been implicated in the pathogenesis of a wide variety of diseases, mostly notably heart, cancer, inflammatory and renal diseases [18] [19]. When skin is exposed to high levels of UV, Photo-oxidative damage is induced by the formation of different types of reactive species of oxygen, super oxide, radicals and peroxide radicals. These forms of reactive oxygen damage cellular lipids, proteins and DNA and cause premature aging of the skin, photo dermatoses and skin cancer [20], based upon these observations, and results, and in search for new antioxidants substances as 6-azauracil derivatives have been synthesized in view of their antioxidant effects.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was used to produce and reducing the odd electron stable-free radical which showed a strong UV-absorption maximum at λ = 517 nm. The new systems obtained dissolved in DMSO/EtOH at 50 and 300 mmol·L−1 added to DPPH at 100 mmol·L−1.
The tube kept at room temperature for 20 minutes and the absorption measured at λ 517 nm. The difference between the test and the control taken as the present scavenging of the DPPH radical by the formula
where AB = Absorption of blank, AA = Absorption of the tested compound.
The radical scavenging activity of ascorbic acid also measured and compared with that of the difference synthesized compound AA [21] - [26]. The results obtained as shown in Table 2. From this data, we can conclude that:
![]()
Table 2. The DPPH radical scavenging activity of novel N-substituted 6-azauracils at 150 and 300 mmol·L−1.
1) The activity higher in the order 8 > 10 > 6 > 7 > 9 > 13 > 12 > 16.
2) In view of the activity of a new compound which attribute to compounds 8 to sulfanilamide drug 6 and 10 to sulfonic acid while compounds 6 and 8 to presence of Mannich bases. Also, the activity of compound 9 attribute to presence of 4-aminoantipyrine drug, which compounds 13 and 12 due to bonded with thiourea and urea moieties.
3) It is clear that the antioxidant activities of these compounds depend on the distribution of electrons nature over all the 6-azauracil derivatives.
4) All the results obtained agree with the other study on the substituted 6-azauracil.
6. Conclusion
In search for new antioxidant probs, some new 1,3-disubstituted-5-spiro(florin-9’-yl)-6-azauracils have been obtained via a simple and safe alkylation and formation followed by condensation with amines. Most of the new targets exhibit a degree of antioxidant activity in the compare with ascorbic acid by using DPPH to produce and reduce the stable odd-electron free radical.