Association between Fetal Heart Rate Monitoring during Labor and Neonatal Acidosis in Full-Term Newborns: A Retrospective Multicenter Cohort Study ()
1. Introduction
Neonatal acidosis is defined as an umbilical cord blood pH below 7.15 at birth [1]. Neonatal asphyxia for its part, is characterized by a metabolic acidosis (pH < 7.00 and base excess (BE) < 12 mmol/L) associated with clinical criteria such as a 5-minute Apgar score < 7 or a sign of neonatal encephalopathy. Current clinical data indicate that the risks of neonatal complications are significantly higher for arterial cord blood pH values below 7.00 [2]. However, the prognosis for those children is extremely different; indeed, some will have a favorable neurological prognosis, whereas others will develop neonatal hypoxic ischemic encephalopathy, despite an appropriated treatment with therapeutic hypothermia. Perinatal hypoxemic ischemic encephalopathy is a major cause of neonatal encephalopathy and is associated with neurodevelopmental impairment and contributes to infant mortality [3].
Intrapartum fetal heart rate (FHR) monitoring is a diagnostic tool to assess fetal well-being and to predict the acid-base status of newborns. Although this non-invasive technique is routinely used during labor, its ability to reduce neonatal morbidity and mortality is still a question of debate [4]. In a large review conducted by the Cochrane Database, the authors highlight that continuous FHR monitoring during labor is associated with halving of neonatal seizures, but no other clear differences concerning neonatal benefits are observed [5]. Indeed, one of the major limitations of this method is the high inter/intra-observer variability due to subjective interpretations of this test [6].
In 2008, the National Institute of Child Health and Human Development published national guidelines for the interpretation of FHR tracing [7] with a full description of different characteristics that are more or less suggestive of neonatal acidosis severity [8] [9].
The International Federation of Gynecologic and Obstetrics (FIGO) published in 2015 modified guidelines on intrapartum fetal monitoring, and advocates for a three-tiered classification system to qualify FHR patterns: normal, suspicious and pathological [10].
In this context, the main objective of our study was to explore the association between the categories of FHR analysis (according to FIGO classification) and neonatal acidosis (severe versus non-severe) in full-term newborns. Our secondary objective was to investigate whether some FHR characteristics during the active phase of labor could predict the severity of neonatal acidosis.
2. Methods
2.1. Study Design and Population
This is a multicenter retrospective cohort study carried out between January 2014 and December 2018. It was conducted in 6 maternity units in the Champagne Ardennes region including Reims University Hospital and other maternity units of different levels. Any child born and hospitalized in a pediatric intensive care unit in one of the participating hospitals was identified retrospectively. There was no specific coding for “neonatal acidosis”. Therefore, we searched and included children hospitalized with an ICD-10 coding type “P91.6” corresponding to “Hypoxic Ischemic Encephalopathy”, with the consequence of losing exactness. We excluded children born before 37 weeks because characteristics of those children and prematurity are known to influence neonatal acidosis.
2.2. Data Collection
A data chaining was then performed to retrieve maternal data from the pediatric records. Specific collections of information from the medical file have been realized for each patient. The data from the medical record included maternal characteristics, as well as pregnancy and delivery parameters. Information on fetal characteristics at birth has been collected from the pediatric record.
The parameters studied were:
· Demographic parameters:
Maternal age, weight, smoking, gravidity and parity, past-cesarean delivery, history of neonatal death.
· Pregnancy and obstetrics characteristics:
Gestational diabetes mellitus, hypertensive disorders, preeclampsia, premature rupture of membranes, suspected intra-uterine growth restriction, fetal macrosomia.
· Delivery characteristics:
Gestational age (weeks), mode of delivery, instrumental delivery induction of labor, duration of expulsive efforts, fetal presentation.
· Interpretation of fetal heart rate monitoring:
Classification according to FIGO guidelines, fetal tachycardia, uterine tachysystole, sinusoidal FHR, decelerations, variability.
· Delivery characteristics and complications:
Birth weight, gender, umbilical cord around the neck, 5-minute Apgar score, arterial umbilical cord pH, lactate, base deficit, pCO2, orotracheal intubation, neonatal death.
2.3. Primary Endpoint
The primary endpoint was to explore whether pathological intrapartum FHR monitoring during the active phase of labor is associated with neonatal acidosis (severe versus non-severe). FHR tracings were analyzed as described in the literature, for 60 minutes before the beginning of the expulsive efforts or the delivery. The classification of FHR during labor was reviewed by an obstetrician and the resident in charge of the study, and was qualified according to the FIGO 2015 nomenclature into three categories: normal, suspicious or pathological cardiotocography (CTG). The category 1 refers to a normal tracing which is predictive of a normal acid-base status, in contrast category 3 called pathological is considered associated with an increased probability of neonatal acidosis at the time of observation. Concerning the category 2, it is considered as a suspicious FHR and not predictive of an abnormal fetal acid-base status but requires regular reevaluation (Appendix A).
The reviewers interpreted FHR tracings without knowing neonatal pH value. We examined the baseline heart rate, variability (normal or less than 5 beats per minute), the presence of decelerations (early, variable, and late decelerations), the presence of fetal tachycardia or bradycardia, the presence of sinusoidal FHR, and uterine tachysystole.
2.4. Regulatory Aspects
In conformity with the French Data Protection Act, a CNIL declaration has been made to the data protection correspondents of each establishment in the study. Patients were informed of their participation in the study and their consent was collected. The different establishments respect the MR004.
2.5. Statistical Analysis
2.5.1. Descriptive Analysis
We first studied the distribution of patients and their characteristics. The continuous variables were expressed as mean and standard deviation, the categorical variables as number and percentage. As appropriate, statistical significance was performed using the Student’s t-tests or Mann Whitney’s U-tests for continuous variables, and Chi-2 tests or Fisher’s exact tests for categorical variables.
2.5.2. Analysis of the Association between Interpretation of FHR According to FIGO Guidelines and Severe Neonatal Acidosis
The association between the interpretation (in categories) of FHR according to the FIGO guidelines and severe neonatal acidosis was tested and quantified by univariate logistic regression. Odds Ratio and 95% confidence intervals (CI) were calculated using the model and presented. We divided the study population into two groups: “severe neonatal acidosis (with an arterial pH < 7.00)” and “non-severe neonatal acidosis (arterial pH > 7.00)”. Multiple imputation with m = 100 imputations was tested, using Rubin rules to combine the results. The analysis was first performed in “complete cases” and then after multiple imputation. The results of both analyses were presented. p < 0.05 was considered statistically significant.
3. Results
We included 55 patients between 1st January, 2014 and 31st December, 2018.
Figure 1 represents the flow chart of patients included in the study. In total, we collected 27 maternal records with severe neonatal acidosis (pH < 7.00), and 28 records with a non-severe neonatal acidosis (pH > 7.00).
3.1. Descriptive Analysis
3.1.1. Maternal and Current Pregnancy Characteristics (Table 1)
Table 1 presents the initial characteristics of the study population, as well as the analysis of parameters comparing severe neonatal acidosis (pH < 7.00) to non-severe neonatal acidosis. The study population was in 36.7% of cases of primiparous patients, and most of pregnancies were singleton pregnancies (98.2%). The two groups were comparable concerning baseline demographic characteristics including maternal age (30.3 vs. 29.1, p = 0.39) and weight (83.5 kg vs. 69.0 kg, p = 0.09). There were no significant differences between the two groups concerning preeclampsia, gestational diabetes mellitus, hypertensive disorders, suspected intra-uterine growth restriction and fetal macrosomia (p > 0.05).
3.1.2. Delivery Outcomes (Table 2)
In the great majority of cases, fetuses were in cephalic presentation (96.4%). The average gestational age at delivery was 39.6 weeks. Almost half the women had an emergency cesarean section (52.7%). The data analysis shows that 55.2% of caesarean sections were performed for fetal heart rate abnormalities. In 17.2% of caesarean sections cases, an “unavoidable” cause of neonatal acidosis had been determined (placenta abruption, cord prolapse). An assisted delivery was required in 21.8% of vaginal deliveries. Almost half of deliveries in each group were carried out by cesarean sections (55.6% vs 50.0%, p = 0.68). The 3 cases of
![]()
Figure 1. Flowchart of patients included in the study.
![]()
Table 1. Maternal and current pregnancy characteristics.
aPercentage calculated from collected data; *Maternal weight at the beginning of the pregnancy; SD, Standard Deviation; IUGR, intra-uterine growth restriction; PROM, premature rupture of membranes.
aPercentage calculated from collected data; *Induction of labor using prostaglandins; FHR, Fetal Heart Rate.
placenta abruption reported were in the severe neonatal acidosis group (20% vs 0%, p = 0.24). In addition, FHR abnormalities were the main indication for caesarean section in both groups (53.3% vs 57.1%, p = 0.24).
3.1.3. Newborn Characteristics (Table 3)
Newborns were more often female (56.4%). The mean arterial pH was 7.02 (SD 0.18), the majority of fetuses had to be intubated (81.8%) and 10.9% of them died after birth. The mean arterial pH was significantly lower in the severe neonatal acidosis group (6.86 vs. 7.15, p < 0.001).Six newborns in the severe neonatal acidosis group died after birth (22.2% vs. 0%, p = 0.01). Newborns with severe neonatal acidosis presented a base excess at birth (−16.4 vs. −10.0, p = 0.001) and significantly higher lactate levels (15.4 vs. 12.2, p = 0.05). There was no significant difference in Apgar score and orotracheal intubation between the two groups (p > 0.05).
3.2. Primary Endpoint: Classification of FHR and Neonatal Acidosis (Table 4 & Table 5)
Concerning interpretation of FHR according to FIGO guidelines, nearly 75% of the tracings were classified as “Category 3: pathological”. Most of the FHR tracings categorized as “pathological” belonged to the severe neonatal acidosis group (91.7% vs. 56.5%, p = 0.003) (Table 4). The category 3 “pathological” of the FIGO fetal heart rate classification was significantly associated with an increased risk of severe neonatal acidosis (OR = 8.59 (1.60 - 46.32), p = 0.003) (Table 5).
3.3. Secondary Endpoints: Predictive Factors of Severe Neonatal Acidosis (Table 4 & Table 5)
Reduced variability (57.8%), bradycardia (44.4%), and late decelerations (51.1%) were the most common FHR abnormalities found in our study. There were more fetal bradycardia (59.7% vs 39.4%, p = 0.05) and more late decelerations (31.8% vs 14.0%, p = 0.04) recorded in the severe neonatal acidosis group compared to
aPercentage calculated from collected data; SD. Standard Deviation.
![]()
Table 4. Characteristics of fetal heart rate monitoring during labor.
aPercentage calculated from collected data; FHR, Fetal Heart Rate; bpm, beats per minute.
![]()
Table 5. Association between interpretation of FHR according to FIGO guidelines and severe neonatal acidosis (arterial umbilical cord pH < 7.00). Univariate analysisa.
aUnivariate logistic model; bFull cases analysis; cAnalysis with multiple imputation; bpm, beats per minute.
the non-severe neonatal acidosis group (Table 4).
The presence of decelerations during CTG was also significantly associated with severe neonatal acidosis: variable decelerations led to a significant reduction of severe neonatal acidosis risk (OR 0.21 (0.05 - 0.95), p = 0.04) whereas late decelerations significantly increased this risk (OR 1.20 (0.19 - 7.69), p = 0.04). Fetal bradycardia significantly increased the risk of severe neonatal acidosis (OR 3.29 (0.94 - 11.54), p = 0.05). No significant association was observed between severe neonatal acidosis and reduced variability, fetal tachycardia and sinusoidal FHR (Table 5).
4. Discussion
Our study shows a significant association between severe neonatal acidosis and category 3 “pathological” fetal heart rate interpretation during labor according to FIGO guidelines. FHR analysis presents a good sensitivity but a limited specificity because of its inter- and intra-observer variability [11]. In fact, in a recent study, Clark et al. highlighted that only half of the children born with neonatal metabolic acidosis were identified intrapartum [12]. In this context, it appears essential that guidelines should be simple, objective and reproducible in order to screen situations most at risk of severe acidosis. That is the reason why, in 2015, the FIGO updated its guidelines on interpretation of FHR tracing and emphasized the need for a careful analysis, in order to prevent severe neonatal acidosis outbreak [10].
In our study, late decelerations, which reflect the activation of chemoreceptors in response to fetal hypoxia [13], were significantly associated with severe neonatal acidosis. This result has already been observed in the literature [8] [14] [15]. Indeed, a Spanish case-control study demonstrated that decelerations occurring during the last 30 minutes of labor were the best marker of neonatal acidosis [14].
In addition, we observed that variable decelerations decreased the risk of severe neonatal acidosis. Although this result is not found in the literature, Williams et al. showed that when the FHR variability was normal, the presence of variable decelerations did not lead to severe neonatal acidosis in 97% of cases [16]. Indeed, variable decelerations that are mediated by baroreceptor stimulation constitute most of decelerations during labor (Appendix B); and their association with other FHR abnormalities may be a sign of neonatal acidosis.
According to the literature, we assessed a significant association between the severity of neonatal acidosis and the occurrence of bradycardia during the second stage of labor. Fetal bradycardia is a very good indicator of acidosis but because of its brutality and unpredictability this makes it hardly exploitable for improving practices [17].
On the other hand, we did not find any significant results concerning reduced variability, probably due to the lack of power of our study caused by the limited cases. In fact, it is well described in the literature that reduced variability is often associated with severe neonatal acidosis, mediated by a mechanism of chronic hypoxia and placental hypoperfusion [18] [19] [20]. Similarly, we did not observe any significant results concerning fetal tachycardia or any significant association with uterine contractions.
Although continuous FHR monitoring is considered as a screening tool to predict fetal hypoxia, it does not provide the cause of neonatal acidosis. The latter can occur either after chronic hypoxia or after an acute and severe complication which is considered as “unavoidable”. In this case, even if an appropriate obstetric management is performed, it often leads to severe neonatal acidosis. Actually, the three cases of placenta abruption reported in our study presented with arterial pH below 7.00 at birth. However, in only 17% of C-sections we found severe neonatal acidosis due to those unavoidable causes. Consequently, our results suggest that neonatal acidosis could have been better predicted for most deliveries. In fact, in a nationwide descriptive study, Berglund et al. observed that in 71% of cases of newborns with an Apgar score < 7 at 5 min, obstetrical care was judged to be non-optimal with a delay in medical intervention in front of repeated pathological FHR monitoring [21]. In 52% of cases, there was a medical malpractice at delivery which highlights that medical decisions can contribute to a situation of fetal hypoxia.
Our study has a number of important strengths. First, it is multicenter study which also allows us to explore different levels of maternity in the Grand Est region. The women characteristics included were comparable to the national profile from the French National Perinatal Survey 2016 [22]. Our criteria for severe neonatal acidosis met a very specific definition which is described as being associated with neurological injury [23] [24].
Nevertheless, our study has some limitations. First, the reduced number of included patients (n = 55) leads to a lack of power, making statistical calculations difficult. Therefore, we were unable to perform a multivariate analysis and we could not take into account some confounding biases. Secondly, we do not have a control group to allow matching and comparison to a healthy group. Third, we included only children hospitalized in an intensive care unit for hypoxic ischemic encephalopathy because there is no ICD-10 coding for severe neonatal acidosis. So we inevitably created a selection bias. Finally, the main limitation of our study was the variability between observers for the interpretation of FHR monitoring. A collegial review of CTGs would help to reduce this subjectivity bias.
Our study suggests that a misinterpretation of the FHR tracing can sometimes lead to a delay in obstetrical intervention and increased maternal-fetal risks. In this context, regular and frequent training of obstetrical teams (midwives, residents, doctors) is essential and widely recommended [25]. It seems important to organize mortality and morbidity reviews, review sessions of contentious cases, and evaluations of professional practices to improve neonatal morbidity.
Appendix A: FHR Classification FIGO 2015
FIGO modified Guidelines 2015 on intrapartum fetal heart rate monitoring
*Decelerations are repetitive when associated with >50% contractions. Absence of accelerations during labor is of uncertain significance.
Appendix B: Schematic Illustration of the Scientific Basis for Hypoxemic and Non-Hypoxemic Fetal Heart Rate Decelerations
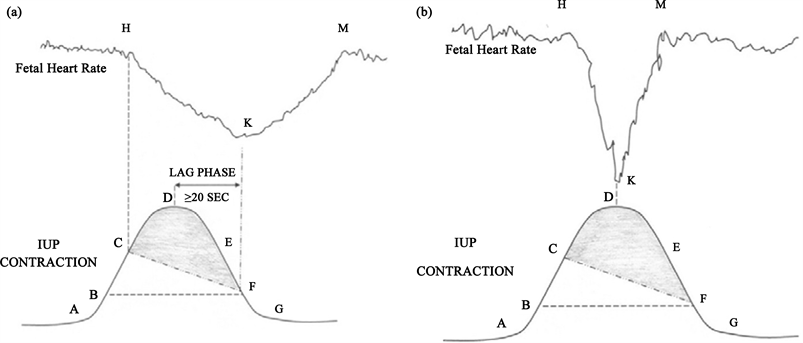
Sholapurkar. Is fetal heart rate “deceleration area” the silver bullet for detection of acidemia? Am J Obstet Gynecol 2018.
(a) Diagram of fetal heart rate deceleration (H-K-M) resulting from peripheral chemo-reflection due to hypoxemia; (b) Diagram of non-hypoxemic variable decelerations resulting from peripheral baroreflection.