Synthesis, Characteristic and Antimicrobial Activity of Some New Spiro[indol-thiazolidon-2,4-diones] and Bis(5-fluorospiro[indoline-3,2’-thiazolidine]-2,4’-dione) Probes ()
1. Introduction
Recently, the chemistry of 4-thiazolidinone derivatives has been subjected to be much attractive for the chemists, due to their using as core-structure in large hetero-polycyclic-sulfur/nitrogen systems covering a wide range of biological, pharmacological, and medicinal application [1] [2] [3] [4] [5]. Moreover, 4-thiazolidinone derivatives were exhibited anticonvulsant [6], hypnotic [7], antitubercular [8], anthelmintic [9] [10], antimicrobial [11], anticancer [12] [13], antihistaminic [14], antifungal [15], anti-inflammatory [16], andantiviral [17], activities. Also, substituted indolones have gained significant attention as a pharma-core unit in the synthesis of more bioactive compounds [18]. On the other hand, spiroindoles, now wish to report the synthesis of some asymmetrical spiroheterobicyclic systems [19] [20] [21] [22]. Interestingly, spiroindoles had significant biological activities such as antiproliferative [23], antibacterial, antifungal [24], anti-inflammatory, fungistatic, bacteriostatic, and anticonvulsant [25] [26]. Based on these observations, the present work tends to obtain novel spiro[indole-thiazolidinones] derived from 5-substituted isatins, sulfanilamide, and thioglycolic acid because of their antimicrobial activity. And benzylpenicillin is used as antibacterial and Imidil (Clotrimazole) as antifungal standards.
2. Experimental
2.1. General
The melting points were recorded on Stuart scientific SMP30 (Bibby, UK) melting point apparatus and reported as uncorrected. A Perkin Elmer model RXI-FT-IR 55,529 cm−1 was used for recording the IR spectra. A Brucker advance DPX 400 MHz using TMS as an internal standard was used for recording the 1H and 13C NMR spectra in deuterated DMSO (δ in ppm) as a solvent. AGC-MS-QP 1000 ex-model was used for recording the mass spectra. Elemental microanalysis was performed on a Perkin-Elmer CHN-2400 analyzer.
2.2. Synthesis of Compounds
4-[5-Nitro/methoxy/fluorophenyl-2-oxo-1H-3-iminoindole-3-yl]benzenesulfonamides (3a-c)
A mixture of 5-nitro/methyl/fluorophenyl isatins (1) (0.1 mol) and sulfanilamide (2) (0.1 mol) in MeOH (200 ml) was refluxed for 2 h, cooled then poured onto ice. The solid obtained was filtered off and crystallized from dioxane to give 3a-c, respectively.
3a: Yield 25.95, 75%, M.p: 268˚C - 270˚C. FT-IR spectrum v (cm−1): 3200 (NH), 3020 (ArH), 1680 (C=O), 1610 (C=N), 1530, 1350 (asymm. and symm. NO2), 1340 (SO2NH2), 900, 860 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.80 (s, 1H, NH), 7.59, 7.56 (m, 2H, ArH), 6.89 (1H, ArH), 7.41 - 7.22 (m, 4H, ArH), 3.83 (s, 2H, NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 196 (C=O), 142 (C=N), 119, 116 (C-C indole), 132 - 122 (aromatic carbons). Calculated, C14H10N4O5S (M+ 346), %: C, 48.55; H, 2.91; N, 16.18; S, 9.26. Found, %: C, 48.24; H, 2.69; N, 16.04; S, 9.17.
3b: Yield 23.17 g, 70%, M.p: 175˚C - 178˚C. FT-IR spectrum v (cm−1): 3180 (NH), 2980, 2850 (aliphatic CH), 1685 (C=O), 1620 (C=N), 1480, 1440 (deform. CH), 1340 (SO2NH2), 1090 (C-O-Me), 850, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.80 (s, 1H, NH), 7.66, 7.64 (2H, ArH), 6.99 (1H, aromatic), 7.41 - 7.22 (m, 4H, ArH), 3.88 (2H, SO2NH2), 3.55 (s, 3H, OCH3). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 168.5 (C=O), 142 (C=N), 140 (C-O-C), 132 - 124 (aromatic carbons), 39.80 (CH3 carbons). Calculated, C15H13N3O4S (M+ 331), %: C, 54.37; H, 3.95; N, 12.68; S, 9.68. Found, %: C, 54.25; H, 3.73; N, 12.59; S, 9.48.
3c: Yield 25.52 g, 80%, M.p: 160˚C - 160˚C. FT-IR spectrum v (cm−1): 3200 (NH), 3050 (aromatic CH), 1690 (C=O), 1610 (C=N), 1340 (SO2NH2), 1250 (C-F), 850, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 13.15 (s, 1H, NH), 7.70, 7.66 (2H, aromatic), 7.40 - 7.22 (m, 4H, aromatic), 3.89 (2H, SO2NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 168.55 (C=O), 145 (C-F), 140 (C=N), 132 - 126 (aromatic carbons), 119, 116 (C-C indole). Calculated, C14H10FN3O3S (M+ 319), %: C, 52.66; H, 3.16; N, 13.16; S, 10.04. Found, %: C, 52.48; H, 3.05; N, 13.01; S, 9.89.
4-[5-Nitro/methoxy/fluorophenyl-1-(4’-nitrobenzoyl)-2-oxo-3-iminoindole-3-yl]benzene sulfonamides (4a-c)
A mixture of 3a-c (0.06 mol) and 4-nitrobenzoyl chloride (0.06 mol) in DMF (150 ml) was refluxed for 1 h, cooled, then the poured onto ice. The solid produced was filtered off and crystallized from dioxane to give 4a-c respectively.
4a: Yield 21.38 g, 72%, M.p: 242˚C - 244˚C. FT-IR spectrum v (cm−1): 3060 (ArH), 1700, 1680 (2C=O), 1610 (C=N), 1530, 1320 (asymm. and symm. NO2), 1340 (SO2NH2), 880, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.88, 7.82, 7.78, 7.72 (each dd, 4H, ArH), 7.42 - 7.22 (m, 4H, aromatic), 3.85 (s, 2H, NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 170, 165.8 (2C=O), 142 (C=N), 140 (C-N), 132 - 122 (aromatic carbons). Calculated, C21H13N5O8S (M+ 495), %: C, 50.91; H, 2.65; N, 14.14; S, 6.47. Found, %: C, 50.79; H, 2.53; N, 14.07; S, 6.32.
4b: Yield 21.02 g, 73%, M.p: 165-167˚C. FT-IR spectrum v (cm−1): 3060 (ArH), 1700, 1680 (2C=O), 1618 (C=N), 1530, 1350 (asym, and symm. NO2), 1340 (SO2NH2), 910, 880, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.88, 7.82, 7.77 - 7.75 (each dd, 4H, aromatic), 7.40 - 7.22 (m, 4H, aromatic), 3.85 (2H, SO2NH2), 3.55 (s, 3H, OCH3). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 170, 168 (2C=O), 142 (C=N), 140 (C-N), 132 - 124 (aromatic carbons), 39.19 (CH3 carbons). Calculated, C22H16N4O7S (M+ 480), %: C, 55.00; H, 3.36; N, 11.66; S, 6.67. Found, %: C, 54.89; H, 3.29; N, 11.48; S, 6.61.
4c: Yield 21.34 g, 76%, M.p: 183˚C - 185˚C. FT-IR spectrum v (cm−1): 3050 (aromatic CH), 1705, 1688 (2C=O), 1610 (C=N), 1530, 1320 (asym. and symm. NO2), 1345 (SO2NH2), 1250 (C-F), 1100 (C-O-C), 880, 850, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.85 - 7.82, 7.78 - 7.67 (each dd, 4H, aromatic), 7.60 - 7.42, 7.40 - 6.99 (m, 4H, aromatic), 3.88 (2H, SO2NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 172, 169 (2C=O), 145 (C-F), 142 (C=N), 140 (C-N), 132 - 122 (aromatic carbons). Calculated, C21H13FN4O6S (M+ 468), %: C, 53.85; H, 2.80; N, 11.96; S, 6.84. Found, %: C, 53.76; H, 2.59; N, 11.78; S, 6.81.
Spiro[indol-thiazolidon-2,4-diones] (6a-c)
A mixture of compounds 4a-c (0.02 mol) and thioglycolic acid (0.02 mol) in dioxane (100 ml) was refluxed for 8 h, cooled then poured onto ice. Then the solution was neutralized with aq. Na2CO3. The solid yielded, was filtered off and crystallized from acetone to give 6a-c respectively.
6a: Yield 7.70 g, 67%, M.p: >300˚C. FT-IR spectrum v (cm−1): 3060 (ArH), 2990, 2870 (aliphatic CH), 1720, 1700, 1685 (3C=O), 1480, 1440 (deform. CH2), 1530, 1320 (asymm. and symm. NO2), 1340 (SO2NH2), 1150 (C-S-C), 870, 850, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.88, 7.80, 7.77, 7.72 (each dd, 4H, ArH), 7.41 - 7.21, 7.10 - 6.99 (each m, 6H, ArH), 4.97 (s, 2H, CH2S), 3.85 (2H, SO2NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 169, 165, 158 (3C=O), 142 (C=N), 140 (C-N), 132 - 124 (aromatic carbons), 70.9 (CH2 carbons). Calculated, C23H15N5O9S2 (M+ 569), %: C, 48.51; H, 2.65; N, 12.30; S, 11.26.Found, %: C, 48.34; H, 2.46; N, 12.07; S, 11.06.
6b: Yield 7.53 g, 68%, M.p: 288˚C - 290˚C. FT-IR spectrum v (cm−1): 3050 (ArH), 2980, 2880 (aliphatic CH), 1720, 1700, 1681 (3C=O), 1531, 1325 (asym, and symm. NO2), 1340 (SO2NH2), 1080 (C-S-C), 890, 860, 810 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.88, 7.85, 7.81 - 6.79 (each dd, 2H, ArH), 7.68 - 7.55, 7.41 - 6.99 (each m, 8H, ArH), 4.77 (2H, CH2S), 3.85 (2H, SO2NH2), 3.57 (s, 3H, OCH3). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 172, 168, 161 (3C=O), 140 (C-N), 137 (C-S-C), 133 - 128 (aromatic carbons), 78.88 (CH2 carbons). Calculated, C24H18N4O8S2 (M+ 554), %: C, 51.98; H, 3.27; N, 10.10; S, 11.56. Found, %: C, 51.75; H, 3.08; N, 10.01; S, 11.49.
6c: Yield 7.05 g, 65%, M.p: 228˚C - 230˚C. FT-IR spectrum v (cm−1): 3060 (aromatic CH), 2960, 2870 (aliphatic CH), 1720, 1700, 1688 (3C=O), 1525, 1320 (asym. and symm. NO2), 1342 (SO2NH2), 1470, 1440 (deform. CH), 1220 (C-F), 880, 820, 770 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 7.78 - 7.75, 7.70 - 7.66 (each dd, 2H, ArH), 7.61 - 7.59, 7.55 - 6.45 (each m, 8H, ArH), 4.97 (CH2), 3.79 (2H, SO2NH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 173, 171, 168 (3C=O), 145 (C-F), 140 (C-N), 138 (C-S-C), 133 - 126 (aromatic carbons), 79.9 (CH2 carbons). Calculated, C23H15FN4O7S2 (M+ 542), %: C, 50.92; H, 2.79; N, 10.33; S, 11.82. Found, %: C, 50.86; H, 2.58; N, 10.17; S, 11.57.
3,3’-(1,4-Phenylenebis(azaneylylidene))bis(5-fluoroindolin-2-one) (7)
A mixture of 5-fluoroisatin (0.06 mol) and 1,4-diaminobenzene (0.03 mol) in MeOH (150 ml) was refluxed for 1 h, cooled. The solid yielded, was filtered off and crystallized from EtOH to give 7. Yield 21.22 g, 88%, M.p: 278˚C - 280˚C. FT-IR spectrum v (cm−1): 3150 (NH), 1670 (C=O), 1250 (C-F), 880, 840 (substituted phenyl). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.90 (s, 1H, NH), 8.55 - 8.35 (HC-CF and HC-C-CF), 7.77 - 7.75, 7.71 - 7.68 (each dd, 2H, ArH). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 186 (C=O), 148 (C=N), 140 (C-F), 128 - 122 (aromatic carbons). Calculated, C22H12F2N4O2 (M+ 402), %: C, 65.67; H, 3.01; N, 13.92. Found, %: C, 65.39; H, 2.86; N, 13.71.
3’,3’’’-(1,4-Phenylene)bis(5-fluorospiro[indoline-3,2’-thiazolidine]-2,4’- dione) (8)
A mixture of compound 7 (0.04 mol) and mercaptoacetic acid (0.16 mol) in dry dioxane (100 ml) was refluxed for 8 - 10 h, cooled then treated with aq. K2CO3. The solid produced was filtered off and washed with cold water then crystallized from acetone to give 8. Yield 13.64 g, 62%, M.p: 188˚C - 190˚C. FT-IR spectrum v (cm−1): 3180 (NH), 1660 (C=O), 1480, 1440 (deform. CH2), 1220 (C-F). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.93 (s, 1H, NH), 8.54 - 8.32 (HC-CF and HC-C-CF), 7.71 - 7.69, 7.67 - 7.65 (each dd, 2H, ArH), 3.98, 3.90 (dd, 4H, 5-CH2). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 183, 170 (2C=O), 142 (C-F), 135 (C-S), 130 - 123 (aromatic carbons), 76 (S-C-N), 33.9 (5-CH2). Calculated, C26H16F2N4O4S2 (M+ 550), %: C, 56.72; H, 2.93; N, 10.18; S, 11.65. Found, %: C, 56.64; H, 2.84; N, 10.07; S, 11.57.
3’,3’’’-(1,4-Phenylene)bis(5-fluoro-5’-(2,2,2-trifluoroacetyl)spiro[indoline-3,2’-thiazolidine]-2,4’-dione) (9)
A mixture of compound 8 (0.02 mol) and 2,2,2-trifluoro acetic anhydride (0.04 mol) in THF (100 ml) was refluxed for 2 h, cooled. The solid produced was filtered off and washed with cold water, dried. Then it was crystallized from dioxane to give 9. Yield 10.38 g, 70%, M.p: 178˚C - 180˚C. FT-IR spectrum v (cm−1): 1730, 1690, 1670 (3C=O), 1220 (C-F). 1H NMR (400 MHz, DMSO-d6) δ (ppm): 8.93 (s, 1H, NH), 8.51 - 8.29 (HC-CF and HC-C-CF), 7.77 - 7.50 (each dd, 2H, ArH), 5.10 (s, 1H, 5-CH). 13C NMR (400 MHz, DMSO-d6) δ (ppm): 180, 171, 162 (3C=O), 140 (C-F), 136 (C-S), 132 - 122 (aromatic carbons), 68 (S-C-N), 33.9 (5-CH). Calculated, C30H14F8N4O6S2 (M+742), %: C, 48.52; H, 1.90; N, 7.55; S, 8.63. Found, %: C, 48.46; H, 1.83; N, 7.34; S, 8.48.
2.3. The Antimicrobial Evaluation
The effect of the newly synthesized compounds 6a-c and 9 against Escherichia coli and Bacillu ssubtilis bacteria strains and Aspergillus flavus and Aspergillus niger fungi were evaluated as the reported method [27]. Benzylpenicillin (antibacterial) and Imidil (antifungal) were used as the standard antibiotics and used DMSO as solvent and control, under the concentration of 100 μg/ml of each compound. The growth inhibition calculated in each case concerning control [28] [29]. Nutrient agar medium used for growing bacteria while Czapeck’s medium used for growing fungi and incubated lasted for 48 h at 37˚C for bacteria and seven days at 28˚C for fungi. The results obtained, recorded in (Table 1).
![]()
Table 1. The IC50 values at 100 μg/ml of the selected compounds.
Generally, the new compounds 6a-c have exhibited activity towards the tested bacteria and fungi, which may be this activity refers to the presence of benzenesulfonamide, nitroaroyl, and both indole and thiazolidinone moieties. Only the compound 6a showed high activity against the bacteria because this compound has two nitro groups. In contrast, the compound 6c recorded activity against both bacteria and fungi, which maybe refers to the effects of F-atoms, nitro group, indole, and thiazolidinone moieties. Compound 9 was showed the more active of the previous compounds 6a-c because it has the C-F, CF3, indole, and thiazolidinone moieties that perhaps increase its activity against bacteria strains and fungi.
3. Results and Discussions
The uses of heterocycles containing Sulfur, Nitrogen, and Oxygen elements as chemical fertilizers are to enhance the yield of crops and to eliminate all kinds of parasites able to attack the cultivation is becoming important due to the vital problem facing the world to provide foods to an increasing population [22]. Among these heterocycles, were 4-thiazolidinone and isatin derivatives. The present work aim is to obtain some new spiroindol-thiazolidin-2,4-diones in one system to enhancing their biological effects. Thus, condensation of 5-(nitro/methoxy/fluoro)isatins (1a-c) with sulfa-drug, namely, sulfanilamide (2) in refluxing methanol, yielded the 3-imino-indol-2 (1H)ones (2a-c). Nitroaroylation of compounds 3 by warming with 4-nitrobenzoyl chloride in DMF, produced the 1-(4’-nitrobenzoyl)-3-imino-5-(substituted)-indol-2-ones (4a-c) respectively (Scheme 1).
Cyclocondensation reaction of compounds 4 with thioglycolic acid in refluxing
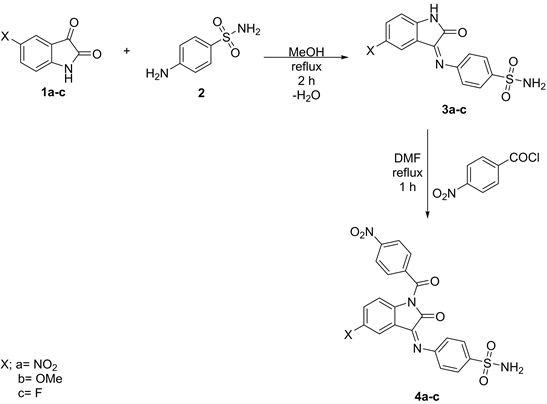
Scheme 1. Formation of compounds 4 from compounds 1 & 2.
non-polar solvent (dioxane) led to the direct formation of spiro [indolthiazolidin-2,4-dione] derivatives 6a-c (Scheme 2). The structure of compound 6 may take place via the formation of the intermediates 5 (not isolated) followed by removal one mole of H2O (Scheme 2).
It is interested that, condensation of 5-fluoroindoline-2,3-dione (1c) with 1,4-diaminobenzene (2:1 by mol) in MeOH yielded the 3,3’-(1,4-phenylenebis (azaneylylidene))bis(5-fluoroindolin-2-one) (7) which upon cycloaddition reaction with excess of mercaptoacetic acid in dry dioxane led to the formation of 3’,3’’’-(1,4-phenylene)bis(5-fluorospiro[indoline-3,2’-thiazolidine]-2,4’-dione) (8). Warming of 8 with 2,2,2-trifluoroacetic anhydride in THF produced 3’,3’’’-(1,4-phenylene)bis(5-fluoro-5’-(2,2,2-trifluoroacetyl)spiro[indoline-3,2’-thiazolidine]-2,4’-dione) (9) (Scheme 3).
The structures of new targets were deduced from their corrected analysis and spectral measurements. The FT-IR spectra of compounds 3a-c were recorded
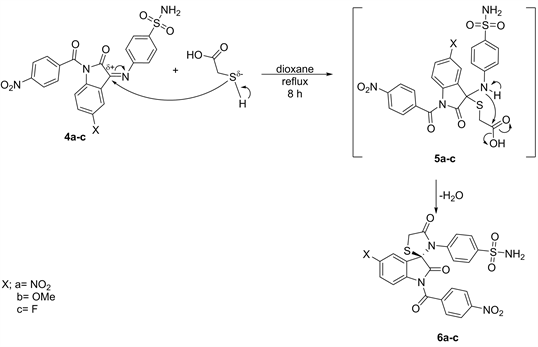
Scheme 2. Formation of compounds 6 from compounds 4.
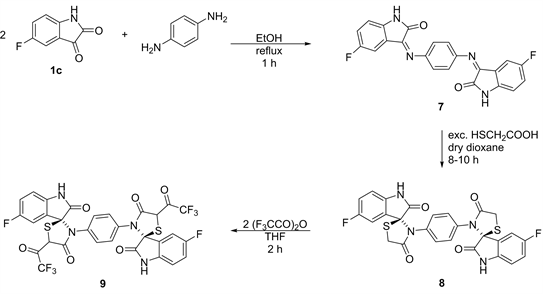
Scheme 3. Formation of compounds 7 - 9.
frequency bands at 1610 cm−1, mainly for exocyclic C=N, and 3150, 1680, and 1340 cm−1 for NH, C=O, and SO2 groups. Additionally, ν at 1530, 1350 cm−1 of asymm. and symm. NO2 for 3a, 1080 cm−1 of C-O-C for 3b, and 1220 cm−1 for C-F of 3c. Also, FT-IR spectra of 4a-c were showed an additional ν at 1710 cm−1 for C=O of 4-nitroaroyl with lacks NH original band. The FT-IR spectra of 6a-c were exhibited new vibrational bands at 2980, 2880, and 1730 cm−1 attributed to aliphatic CH2 and C=O of thiazolidin-4-one moiety with lacks exo C=N of compound 4 which confirmed that cycloaddition reaction has occurred. All the compounds 6a-c were recorded ν at 1530 and 1330 cm−1 for NO2 of 4-nitroaroyl with ν at 3050 and 900 - 800 cm−1 attributed to aryl and phenyl substituted. Moreover, 1H NMR spectra of the synthesized compounds give us a good indication of these structures. Thus, 1H NMR spectra of compounds 3a-c were recorded δ at 13.48 ppm for the NH-indole, with 7.56, 7.46, and 6.89 ppm for indole proton, besides, δ at 7.40, 7.31, and 7.11 ppm for aromatic protons of sulfanilamide, and δ at 3.85 ppm attribute to NH2SO2.1H NMR spectra of compounds 4a-c were showed a lacks δ in the region of 14 - 13 ppm for NH-indole, which that of 6a-c exhibited δ at 4.00 - 3.95 and δ at 3.66 ppm for CH2 of thiazolidinone and SO2NH2 protons. All the aromatic CH-adjacent of NO2, OCH3, F, and SONH2 have appeared as a dd with the coupling constants J1 = 8.3 and J3 = 6.5 Hz. Moreover, 13C NMR spectra of compounds 3a-c were recorded δ at 140 ppm for exocyclic C=N with 169, 145, and 132 - 122 ppm attributed to C=O, C-F, and aromatic carbons, while that for 6a-c showed δ at 39.8 ppm for CH2 carbons of thiazolidinone.
The former structures of compounds 7 - 9 have been established from their corrected elemental as well as spectral studies. FT-IR spectra of compounds 7 - 9 were recorded lacks NH2 functional groups, while compound 8 showed bands at 2900, 2880, and 1480, 1440 cm−1 of CH2 of thiazolidinone formed. These bands were disappeared in compound 9, which confirmed that fluoroacylation reaction occurred. 1H NMR spectrum of compound 8 was recorded resonated signals at δ 3.98 - 3.90 ppm characteristic for active methylene presented, while that for compound 9 showed δ at 5.10 ppm for 5-CH. Finally, the 13C NMR spectra of compounds 7 - 9 were showed δ at 186 - 180 ppm attributed to C=O for the indolinone ring. In contrast, compounds 8 and 9 exhibited δ at 171 - 170 ppm for thiazolidinone; only compound 9 has δ at 162 ppm for C=O of F3C-C=O group.
4. Conclusion
In the search for new heteropolycyclic systems that have high activity against the microbes, some new spiro[indol-thiazolidon-2,4-diones] derived from sulfa drug and substituted isatin have been synthesized and evaluated as antimicrobial probes, where the systems containing fluorine atoms exhibited high activity than the other substituents.