Jackfruit Seeds Protein Isolate by Spray Drying Method: The Functional and Physicochemical Characteristics ()
1. Introduction
The demand for plant-derived proteins is continuously increasing for its’ superior health benefits, easy digestibility and economic rationales [1]. As a consequence, isolation and characterization of cereal and bean proteins such as soy protein, flaxseed protein, and lentil protein have received good attention from the researchers [2] [3] [4]. In the journey of exploring plant proteins, recently, jackfruit seeds (JFSs) have been established as a good source of protein. The protein content of jackfruit seed flour was determined as 16.01% by [5] and 13.50% by [6].
Jackfruit (Artocarpus heterophyllus Lam) belonging to the Moraceae family grows abundantly in south-east Asian countries such as Indonesia, Bangladesh, and India as well as in some areas of Brazil and Australia. Jackfruit is termed as the national fruit of Bangladesh. The annual production of this fruit is more than a million metric tons in Bangladesh, such as in the year 2015, about 1,031,316 metric tons of jackfruit was produced from 27,316 acres of land [7]. A single fruit may contain up to 500 juicy cells, each of them enclosing a seed; thus the whole seeds consist around 10% to 15% of a fruit weight. Traditionally, the majority of the seeds are discarded in the environment, although a little portion is consumed after minimal processing like roasting and boiling. These seeds can, however, be used as a good source of protein, if processed suitably. A significant contribution is possible in meeting the protein demand by using the JFSs protein fraction in food formulations. Understanding of functional and physicochemical properties of isolated and dried protein provides better insights of it hydrothermal behavior. Such information makes it convenient to use in new food formulation.
Isolated proteins are increasingly being used as an ingredient in preparation of the protein enriched foods. Prior information regarding the functional and physicochemical properties of an isolated protein helps to use it in new food formulation with minimum hassle. For better application as an ingredient, the proteins should ideally possess several desirable functional properties such as solubility, water holding capacity, gel formation ability and foaming capacity. These functional properties influence in determining the food characters during processing, storage, and consumption [8]. For example, the higher solubility of a protein increases the foaming and emulsion ability of protein, thus improves the food structure and enhances the digestibility of protein-enriched foods [9]. Knowledge of the emulsifying and foaming properties of proteins is necessary to evaluate their potential to use as food additives. Several molecular parameters such as pH, ionic strength, and viscosity are essential determinants in the formation, stability, and textural properties of protein-fat-water emulsions [10]. The crystalline behavior of any food ingredient determines the critical characteristic of finished product. For example, the amorphous particles possess higher solubility and dispersibility in the solution than the crystalline particles [11]. Similarly, morphology of the particles has great influence on the handling, transportation and storage behavior of the finished product.
The secondary structure of protein plays a vital role in protein folding and unfolding process. The 3D structure of the protein, which is crucial for protein functionality, is attributed to compact and stable secondary structural elements such as α-helix, β-sheet, and β-turn. These elements also play decisive roles in determining evolution, size, and geometry of protein [12]. Fourier Transform Infrared (FTIR) spectroscopy provides information about the secondary structure of proteins by chemical composition and physical state of the sample geometry [13]. The denaturation of protein is an inevitable structural change due to external stresses such as extreme high or low heat and extreme acidic or alkaline conditions. The denatured protein results in poor solubility and lacks in exerting appropriate functionalities [14]. Application of the numerical software for fitting the bands of the spectra can assess the stress exerted denaturation of protein [13] [15].
Various drying methods are used to dry the liquid slurry in the protein isolation process. Spray drying and freeze drying are by far the most popular methods for drying protein solutions [16]. Although the solid protein obtained from freeze drying has the advantage of storage stability, it is not a direct particle formation method. It requires a secondary procedure to break apart the cake into particles. Spray drying is the most common powder generating method, wherein a liquid feed is rapidly transformed into dried particles. Spray drying is a relatively inexpensive powder forming technology. This is about 1/9 and 1/6 times cheaper in terms of capital and operation costs, respectively, compared to freeze drying [17].
Few research initiatives are found in the literature regarding isolation of jackfruit seeds protein and its’ characterization. They employed different methods for drying the isolated protein. The research groups [18] [19] observed the structural and functional properties of vacuum filtered and dried (at 35˚C) jackfruit seeds protein isolate (JSPI). Another group [20] used simple tray drying at 50˚C for drying the protein concentrate. Few studies were devoted for physicochemical characterization and amino acid profile evaluation of freeze dried JSPI [5] [21]. However, despite possessing the highest probability of commercial convenience of the spray dried protein powder, no scientific study, so far, is reported on the preparation and functional characterization of JSPI using this dryer.
Therefore, this study aims at preparing jackfruit seeds protein isolate by spray drying method. The key functional and physicochemical properties of the powder protein were observed. The protein powder was also studied by infra-red spectroscopy to determine the secondary structural properties.
2. Materials and Methods
2.1. Raw Materials
Jackfruit (Artocarpus heterophyllus Lam.) seeds were collected from the local market of Narsingdi, Bangladesh. These were sun-dried for quick separation of the white outer layers. After cutting approximately 3 × 5 mm size, the seed pieces were dried into a cabinet dryer to bring the moisture content within 10% - 12%. Finally, the seeds were ground and passed through a 75-micron sized sieve to get JFSs flour. The obtained powder was packed into airtight containers for further analyses.
2.2. Preparation of Jackfruit Seeds Protein Isolate (JSPI)
JSPI was prepared according to the method followed by [4] with slight modification. The seeds flour was suspended in distilled water (1:10 w/v). The pH of the slurry was adjusted to 9.0 by using 1 M NaOH solution and kept in rest for one hour. The slurry was then centrifuged (at 12,600 g, for 15 min). The supernatant protein solution was separated and adjusted to pH 4.5 using 1 M HCl. The solution was then stirred for 30 min at 400 rpm and left undisturbed for cold precipitation overnight (4˚C). The supernatant was carefully siphoned off, and the obtained protein precipitate was collected by centrifugation at 2217 g for 15 min. The accumulated protein was washed 3 - 4 times with distilled water to eliminate all soluble components. The protein was re-suspended (10%, w/v) in distilled water and the pH was set to 7.0. The protein solution was then dried using a spray dryer (Yamato ADL-311S) to get dry protein powder. The input temperature was 160˚C, the output temperature was 60˚C, and the nozzle air pressure was 0.2 MPa. The spray dried powder was then stored at room temperature in a glass bottle.
2.3. Determination of Protein Content
The protein content of JSPI was determined by the Micro-Kjeldahl method [22] using 6.25 as the protein conversion factor. One (1) g of protein powder was used for digestion, distillation, and titration following the standard protocols for Micro-Kjeldahl method. The average values from the replicated experiments were accepted.
2.4. Determination of Functional Properties of JSPI
2.4.1. Protein Solubility
The solubility of JSPI was determined according to the method used in [23] with slight modification. One (1) g of JSPI powder was diluted into 100 mL of distilled water. The suspension was mixed evenly through continuous stirring for 30 min at 500 rpm. The suspension was then kept in the refrigerator (4˚C) for overnight and centrifuged (12,600 g/20min). The soluble protein content in the supernatant was determined by the Micro-Kjeldahl method [22]. The protein solubility (PS) was estimated from the amount of protein found in the supernatant and the total sample used to prepare the solution following Equation Number 1.
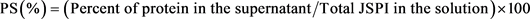 (1)
(1)
2.4.2. Water Holding Capacity
The water holding ability of JSPI was determined according to the method described in [24]. One (1) g of protein sample was suspended into 10 mL of distilled water in a 15 mL graduated conical centrifuge tube. The suspension was stirred evenly at 400 rpm and allowed to stand at room temperature for 1 h. It was then centrifuged at 2217 g for 30 min, and the volume of the supernatant was measured. The water-holding capacity was expressed from the amount of water held by 1.0 g of protein sample.
2.4.3. Oil Holding Capacity
The oil holding capacity of JSPI was determined following a similar method for water holding capacity, where corn oil was used as a suspension medium instead of water.
2.4.4. Bulk Density
Five (5.0) g of JSPI was put into a 25 mL measuring cylinder. The initial weight and the initial volume of the sample were recorded. The JSPI powder was poured into a cylinder and tapped continuously until a constant level was obtained. Again, the weight and volume of the sample were recorded. The final weight and volume of the sample were recorded from these differences. The bulk density (g/mL) was calculated as the weight of powder (g) divided by the volume of powder (mL) according to the Equation Number 2.
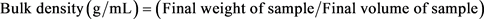 (2)
(2)
2.4.5. Gelation Characteristics
The gelation capacity of JSPI was determined according to the method followed by [25] with a slight modification. A range of sample suspensions from 2% to 14% (w/v) concentrations was prepared into two different solvents; distilled water and 1.0 M NaCl solution. The test tubes containing these suspensions were then heated for 1 h in a boiling water bath and followed by a rapid cooling under cold water tap. The tubes were further cooled for 2 h at 4˚C. The least gelation concentration (LGC) was determined as the minimum concentration required to form a self-supporting gel when the sample did not fall or slip from the inverted test tube.
2.4.6. Measurement of Foam Properties
Foaming capacity and stability of JSPI at different pH (1.5 - 11.5) were determined according to the method described in [26]. The protein samples (2 g) were kept into 250 mL beakers and diluted with 100 mL of distilled water. The pH values were adjusted from 1.5 to 11.5 by using HCl and NaOH. The suspensions were mixed thoroughly using magnetic stirrer and finally homogenized at 5000 rpm for 5 min (WiseMixTM, HG-15 D). The volume of the produced foam in each beaker was measured by measuring cylinder within no later than 30 s. The increment of foam volume was estimated following Equation Number 3 and expressed as percent foam capacity. The foam stability of JSPI was calculated by Equation Number 4 from the decreased foam volume after 30 min.
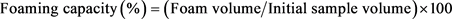 (3)
(3)
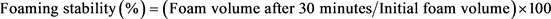 (4)
(4)
2.4.7. Measurement of Emulsion Properties
The emulsification capacity of JSPI was also determined at a range of pH from 1.5 to 11.5 was according to the method stated by [23] and was expressed as percent of oil emulsified per g of protein. A dispersion of 1 g isolated protein in 25 mL distilled water was prepared by continuous stirring at moderate speed for 30 min at 600 rpm. Five (5) mL refined groundnut oil was added, and after adjusting pH the mixed slurry was blended at 3000 rpm until phase separation was seen. The emulsion capacity was estimated based on separated cream following Equation Number 5.
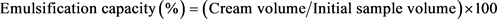 (5)
(5)
The emulsion stability was determined according to the method used in [27] with a slight modification. The above emulsions in different pH were transferred into test tubes and held at 70˚C in a water bath for 45 min. The tubes were then allowed to stand at room temperature for 3 h. Percent stability was calculated from the height of the remaining emulsified layer and the original emulsified layer, according to Equation Number 6.
 (6)
(6)
2.5. Physicochemical Characteristics of JSPI
2.5.1. Particles Size Distributions
The particle size distribution of the spray dried JSPI powder was determined according to [28] with slight modification. The experiments were carried out using a Zeta Sizer (ZEN3600, Malvern Instruments Ltd., Malvern, UK). Well dispersed samples at 4 mg/100 ml (w/v) concentration were used in this test. Dispersions were prepared in distilled water with gentle stirring for 60 min (400 rpm) at ambient temperature. These dispersions were kept overnight at 4˚C for complete hydration and then diluted again with distilled water to the final concentration (4 mg/100 ml, w/v). The size distribution data were obtained and analyzed by using Malvern’s proprietary software version 7.01.
2.5.2. X-Ray Diffraction (XRD) Analysis
The X-ray diffraction (XRD) pattern of JSPI powder was measured to assess the crystalline/amorphous behavior of the isolated protein. This study was carried out on a Siemens (D501) X-ray diffractometer with CuKα1 radiation [29]. Diffractograms were recorded between 8˚ and 52.15˚ (2θ) at a rate of 1.20˚/min (2θ) with a step size of 0.05˚ (2θ). An anti-scatter slit of 0.15 mm and 1˚ divergence and receiving slits were used.
2.5.3. Scanning Electron Microscopy (SEM)
Particle morphology of the spray dried JSPI was observed by using a scanning electron microscope (JEOL, JSM 6300 SEM, JEOL, Tokyo, Japan). The powder sample was directly deposited on an aluminum stub using double-sided adhesive carbon conductive tape. The entire assembly was put in a desiccator containing freshly dried silica gel for 72 hours. Then the sample was sputter-coated with a thin layer of gold. The SEM micrographs were acquired at an accelerating voltage of 15 kV.
2.5.4. Differential Scanning Calorimetry (DSC) Method
DSC tests were carried out to measure the calorimetric changes in spray dried JSPI and these changes were correlated to determine its denaturation temperature. The analysis was carried out by using DSC Q2000, (TA instruments, New Castle, USA) [30]. The JSPI powder was dispersed at 20% (w/w) concentration distilled water. About 18 ± 1 mg of sample solution was taken in the aluminum pan and sealed hermetically. The samples were scanned from 20˚C to 100˚C at a heating rate of 5˚C/min. The instrument was calibrated by using indium standard (fusion temperature = 156.61˚C and fusion enthalpy = 28.67 J/g) [31]. The software associated with the instrument (TA Universal Analysis 2000TM) was used to analyze the DSC thermograms. The extent of denaturation was determined using a change of enthalpy (ΔH) from 50˚C to 95˚C.
2.5.5. Fourier Transform Infrared (FTIR) Spectroscopy
The FTIR spectroscopy was used in the present study to observe the conformation and secondary structural motifs of the isolated protein. The IR spectra were acquired through Perkin Elmer FTIR (Spectrum-2) instrument operated by CPU32M software. The JSPI powder was scanned within 650 to 4000 cm−1 using a triglycine sulfate (TGS) detector. A total of 8 scans at 4 cm−1 resolution were accumulated at 0.2 cm/sec scanning speed. The JFSs flour and the separated JFSs starch were also scanned to observe the comparative spectra among those materials. The protein sample was diluted in deionized water at 1% concentration (w/v) for the secondary structural quantification. The spectrum of the aqueous protein solution was acquired in the same IR conditions described above. The blank spectrum of water was subtracted from the protein spectrum. The baseline subtracted protein spectra were analyzed by using Perkin Elmer’s proprietary software (Version 10.05.03), and a peak fit software Peak Fit version 4.12 (Sea Solve Software Inc. Framingham, USA). The peak Fit Software was used for quantitative analysis of the secondary structure of the protein. The original spectra (of amide region-I, 1600 - 1700 cm−1) without any smoothing were fitted with Gaussian shape and were analyzed by the local least square (LLS) algorithm. Percentage of secondary structures (α-helix, β-sheets, β-turns, and random coils) was estimated using Equation Number 7 [32] [33].
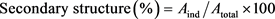 (7)
(7)
where, Aind = Sum of the area of individual secondary structure within amide I band.
Atotal = Area of total amide I band.
The positions or locations of bands for each secondary structural element (β-sheets, α-helix, and β turns) of tested proteins in H2O were considered based on the information available in the literature [12] [34]. The bands from 1620 to 1640 cm−1 and 1674 cm−1 to 1680 cm−1 were assigned to β-sheets. The bands from 1641 to 1647 cm−1 were assigned to random coil. The bands within 1648 and 1660 cm−1 were assigned to α-helix. Similarly, the bands appearing at and in the vicinity of 1663 cm−1, 1671 cm−1, 1683 cm−1, 1688 cm−1, and 1694 cm−1 were assigned to β turns. The peaks between 1600 cm−1 - 1619 cm−1 were not considered while quantifying the secondary structural features as they are known to be generated from aromatic side chains [32].
3. Results and Discussion
3.1. JSPI and Its Protein Content
According to the quantitative assessment using Micro-Kjeldahl method we found that the crude JSPI contained 76.89% protein, which is comparable to other plant based protein isolate. For example, [35] prepared tomato seeds protein isolate with 71.32% protein content, and [36] isolated rice bran protein with 60% protein content. The FTIR spectra, in this study, confirmed the separation of JFSs flour into its two principal components protein and starch (Figure 1). As shown in Figure 1(a), the flour is a mixture of high amount of starch (band region 900 - 1200 cm−1) [37] [38] and comparatively low amount of protein
![]()
Figure 1. FTIR absorbance spectra of (a) JFSs flour; (b) Isolated JFSs protein; (c) JFSs starch after removal of protein.
(band region 1200 - 1700 cm−1) [39] [40]. The spectra b & c on the figure presented the protein and starch isolated from JFSs flour.
3.2. Functional Properties of JSPI
3.2.1. Protein Solubility
The solubility of JSPI was found as 78.44%. This value of solubility is comparable with the percent solubility of other proteins isolated from different sources. Reference [4] found variation in solubility of the protein isolates prepared by different drying methods, such as vacuum dried, spray dried and freeze dried lentil protein isolate showed 51%, 81% and 78% solubility respectively. The literature suggests that the spray dried powder possesses more amorphous particles, which provides increased solubility during rehydration [15] [41]. The percent solubility of rice bran protein concentrate ranged from 47.69% to 73% based on varieties of rice [36]. Protein isolate with higher solubility is preferred for direct consumption as well as utilization as a food ingredient. Therefore, using a spray dryer for commercial production of JSPI is recommended to produce the concentrated protein with higher solubility.
3.2.2. Water Holding Capacity
The water holding capacity of the JSPI was 2.89 mL H2O/g protein, which is higher than the water holding capacity of cowpea protein, 2.20 mL H20/g [42], but lower than that of the wheat bran protein, 4.20 mL H20/g [2]. High water holding capacity of proteins helps to reduce moisture loss from bakery goods and maintains freshness and moist mouth feel of the products. The obtained values indicate that the JSPI powder needs to be mixed with a portion of wheat protein to have adequate swelling and water retention during food preparation.
3.2.3. Oil Holding Capacity
The oil-holding capacity of JSPI was 1.57 mL oil/g protein, which is slightly lower compared to wheat bran protein 1.70 mL/g [2]. Higher oil holding capacity is advantageous for oily food like sausages, mayonnaise, and salad dressing. Therefore, utilization of JSPI combined with a protein of higher oil holding capacity is preferred for such a food.
3.2.4. Bulk Density
This is an important parameter of a powder or a mix of powders to determine the packaging requirement of the product. The bulk density of JSPI was 0.67 g/mL, which is less compared to the bulk density of casein 0.89 g/mL [35]. Low bulk density is advantageous for the formulation of weaning foods regarding packaging and transportation [43].
3.2.5. Gelation Characteristics
In the process of gel formation, about 6% JSPI in 1.0 M NaCl solution initiated forming a gel; however, this gel was not consistent enough to withstand the gravity force at the inverted position of the container. At least 12% (w/v) JSPI concentration in that solution was required to make a firm gel (Table 1). The protein did not form a gel in pure water. Reference [4] found the least gelling concentration of lentil protein isolate was 14% in a phosphate buffer of pH 7.0. The lower least gelation concentration implies the greater gelling capacity of the protein [44].
3.2.6. Foaming Properties
The foaming capacity (FC) of isolated jackfruit seeds protein was pH-dependent. As shown in Figure 2, the alkaline solution resulted in better foam formation. The FC increased from the lowest value of 6% at pH 5.5 to 42%, 25%, 46%, 58%, and 74% at pH 1.5, 3.5, 7.5, 9.5, and 11.5, respectively. The findings comply with the previous observation that the alkaline solution is favorable for the protein solubility and foaming capacity [45]. This trend could be due to the fact that the variation of pH affects the net charge and electrostatic balance of the protein solution. The solubility of protein tends to be minimum near the isoelectric point
![]()
Table 1. Gelation capacity (GC) of jackfruit seeds protein isolate in water and NaCl solution.
Note: Symbols: − no gel; + weak gel; ++ strong gel; +++ very strong gel.
![]()
Figure 2. Effect of pH on the foaming capacity and foaming stability of jackfruit seeds protein isolate (JSPI).
(IP) because of negligible or zero net charge. The IPs of the most plant proteins are reportedly fallen between 4 and 5 [21]. The higher FC at pH 11.5 was likely due to the increased net charges on the protein molecules, which weakened the hydrophobic interactions and increased the flexibility of the protein [2]. These phenomena allowed the protein to diffuse more rapidly to the air-water interface to encapsulate air particles and then enhanced the foam formation.
The foaming stability (FS) requires the formation of a thick, cohesive, and viscoelastic film around each gas bubble [46]. Isolated jackfruit seeds protein showed poor foam stability at pH 5.5 (0.83%). On both sides of pH 5.5, the foaming stability gradually increased and reached its maximum value of 47% at pH 11.5. The solutions with pH 7.5, 9.5, and 1.5 showed about 26%, 39 %, and 21% FSs, respectively (Figure 2). The previous researches also observed a considerable effect of pH on the stability of foams [35] [47]. The improvement of FS in the alkaline pH is likely due to increased solubility and surface activity of the soluble protein.
3.2.7. Emulsifying Properties
The pH of solution has a significant effect on emulsion properties also. As shown in Figure 3, JSPI had a minimum emulsion capacity (EC) (2%) at pH 5.5, which increased on either side of this pH. The pH 11.5 of the solution showed the highest EC of 63%, which was followed by the pH of 9.5 and 7.5, with EC values of 56% and 25%, respectively. The observed results suggest that the alkaline pH improved the emulsion capacity from the acidic one. Dependence of emulsion capacity on pH was expected, as the emulsion capacity of a protein is known to depend on the hydrophilic-lipophilic balance and electrostatic repulsion at the isoelectric point. The JSPI had minimum emulsion stability at pH 5.5 (0.43% after 3 h) and reached the maximum value of 52% at pH 11.5 (Figure 3).
![]()
Figure 3. Effect of pH on the emulsion properties of JSPI.
It is reported that various factors including pH, net charge, interfacial tension, viscosity, and protein conformation could affect the values of ES [48].
3.3. Physicochemical Properties of JSPI
3.3.1. Particle Size and Size Distributions
The particle size and size distribution curve of the spray dried JSPI are presented in Figure 4. The curve suggests that about 75.0% (by volume) particles ranged from 100 to 1000 nm and around 23.0% of the particles were smaller than 100 nm. About 2% particles’ diameter was larger than 1000 nm. The variation of size found in this curve reflects the disparity of morphological image (Figure 6). This observation consents to the previous finding that the micro particulation process of bio particles delivers finer to comparatively larger particles [49].
3.3.2. Crystallinity of JSPI
The XRD diffractogram is presented in Figure 5. Absence of any sharp peak
![]()
Figure 4. Particle size distribution curve of spray dried JSPI.
![]()
Figure 5. X-ray diffraction pattern of spray dried JSPI.
suggests that the spray-dried JSPI particles were amorphous in nature. However, two broad peaks were found at 9.0 and 19.0 degrees 2θ on the JSPI diffractogram. Reference [50] observed three peaks (at 8.5, 19.5 and 24.5 degrees 2θ) on the soy protein isolate diffractogram and reference [4] reported two broad peaks (at 8.5 and 24.5 degrees 2θ) on the lentil protein isolate diffractogram. It is reported that the powder form of protein normally possesses the amorphous behavior [29] [51].
3.3.3. Surface Morphology
The SEM micrographs of JSPI powder presented in two images with two different magnifications appear varying sizes of particles (Figure 6). The surface of the spray-dried protein isolate produced more or less spherical appearance with dimples and surface folds. Higher magnification micrograph shows the smooth-surfaced hollow particles, although they possess indented surface. The low diffusion rate of the moisture from the aqueous droplets containing skin-forming solids is the primary reason for the formation of hollow particles in spray dried particles [52].
3.3.4. DSC Thermograph for Thermal Denaturation of JSPI
According to the DSC thermogram (Figure 7), about 1.810 J/g energy required to denature the jackfruit protein isolate. The endothermic peak produced by the thermal scanning from 20˚C to 100˚C suggests that the denaturation temperature (Td) of JSPI was about 77.83˚C. The denaturation temperature of the spray-dried lentil protein isolate was reported as 123.6˚C [4]. They concluded that the Td value can be changed with the variation of crops or with the verities of same crop. Reference [14] suggested that the variation of Td can be due to the variation of concentration of protein in the solution. They demonstrated that the Td values vary from 75˚C to 80˚C with the variation of concentration of whey protein isolate from 20% to 40%.
![]()
Figure 6. Scanning electron microscope (SEM) pictures of JSPI powders with two different magnifications.
![]()
Figure 7. DSC thermogram of spray dried JSPI (thermal scanning from 20˚C to 100˚C).
3.3.5. Secondary Structural Properties of JSPI
A cut off portion of the amide region-I (1600 - 1700 cm−1) from the original absorption spectrum and its fitted spectra are presented in Figure 8. The amide-I region was selected for secondary structural quantification because of its very high signal to noise ratio [53] [54]. As seen in the figure, there were nine bands (excluding side chain) produced for the best fitting of the amide-I region with an r2 value of 0.97. The quantification tools of the assigned bands estimated that the JFSs protein contained 50.28% β-sheet, 21.71% α-helix, 8.86% β-turn, and 19.15% unordered structure. The result advised that the β-sheet is the dominant secondary structure of JFSs protein.
4. Conclusion
The protein fraction of the jackfruit seeds was successfully isolated through pH treatment, centrifugation and spray drying process. About 77% protein was found to present in the crude JSPI. The isolated protein showed acceptable physicochemical and functional characteristics for the plan of using JSPI in food formulations. For example, it possesses good solubility, amorphous nature, moderate bulk density and an attractive gel-forming ability. Variation of pH in the solution imposed a significant effect on foaming and emulsion properties. Both the foaming capacity and emulsion capacity reached their maximum values at a pH of 11.5. The protein isolate possessed adequate water holding capacity, oil holding capacity, and almost 75% of the particles fall in a similar size distribution range. The JFSs protein contained 50.28% β-sheet, 21.71% α-helix, 8.86% β-turn, and 19.15% unordered properties in the secondary structure. Based on the observed functional and physicochemical features, the JSPI is expected to use
![]()
Figure 8. (a) Absorbance (b) fitted spectra of the JFSs protein isolate in the amide-I region.
as a supplementary ingredient in the food formulations. However, more studies are required to recommend the optimum level of fortification of this protein in foods.
Acknowledgements
The authors gratefully acknowledge the Ministry of Education of Bangladesh for financing the project.
Abbreviations Used
JSPI = Jackfruit seeds protein isolate;
AOAC = Association of Official Analytical Chemists;
FTIR = Fourier-transform infrared spectroscopy;
FC = Foaming capacity;
FS = Foaming stability;
EC = Emulsion capacity;
ES = Emulsion stability;
GC = Gelation concentration;
LGC = The Least gelation concentration;
LLS = Local least square;
TGS = Triglycine sulfate;
JFSs = Jackfruit seeds.