Open Journal of Composite Materials
Vol.04 No.04(2014), Article ID:50816,4 pages
10.4236/ojcm.2014.44023
The Synthesis and Evaluation of a Novel Inhibitor DN for Carbon Dioxide Corrosion
Qingwang Liu, Tong Zhang, Jigang Wang, Zhenzhong Fan, Ao Sun, Dong Cheng
EOR Key Laboratory of the Ministry of Education, Northeast Petroleum University, Daqing, China
Email:LIUQINGWANG@163.com, 648553814@qq.com, wangjigang9999@163.com, fanzhenzhong@163.com, sunsunaoao@126.com, 277134741@qq.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 September 2014; revised 7 October 2014; accepted 17 October 2014
ABSTRACT
During the oil and gas wells exploitation and transportation, carbon dioxide corrosion is one of main reasons for the corrosion of metallic materials. So the methods of preventing and reducing carbon dioxide corrosion have become a widely focused problem. The foreign and domestic practices and experiences show that as an economic, effective and versatile metal corrosion control method, corrosion inhibitor protection technology is suitable for application in oil and gas transportation system. This paper, through the indoor experiment with benzyl chloride, using quinolineas raw materials, synthesizes a kind of quinoline quaternary ammonium salt.The obtained product benzyl chloride quinoline compounded with OP-10 and ethanol to get a novel inhibitor DN for carbon dioxide corrosion, and to evaluate its performance.
Keywords:
Carbon Dioxide, Corrosion Inhibitor, Synthesis Method, Performance Evolution

1. Introduction
In the oil field development, gathering and transportation process, carbon dioxide corrosion problem widely exist. In the proper pressure and humidity conditions, carbon dioxide will produce carbonic acid and it can corrode the oil casing and cement severely. All kinds of metal pipes and equipment will suffer from fierce corrosion and erosion; eventually it will shorten the working life of the pipelines and equipments and cause great economic loss [1] . So the methods of preventing and reducing carbon dioxide corrosion have become a widely focused problem.
The foreign and domestic practices and experiences show that as an economic, simple dosing equipment, easy operation and versatile metal corrosion control method, corrosion inhibitor protection technology is suitable for application in oil and gas transportation system. Now the development of oil and gas well corrosion inhibitor for carbon dioxide corrosion resistance has become more and more important and it can bring huge economic benefits for the oil industry. This experiment will synthesis a novel inhibitor DN for carbon dioxide corrosion with the method of fractional steps and evaluates the performance of the inhibitor.
2. Experimental
2.1.The Experimental Materials and Methods
2.1.1.ExperimentalInstruments
Electric treater(HK/ZX-100); four-necked reaction flask; electric heating-jacket(ZNHW-10,000ml); electronic balance; beakers (JA5003)and so on.
2.1.2.Experimental Materials
Quinoline, analyticalgrade; OP-10, analyticalgrade; Benzyl chloride,analyticalgrade;Ethanol, analyticalgrade; Epoxy chloropropane, analyticalgrade; Acrylic acid, analyticalgrade.
2.1.3.The Synthesis Methods of Corrosion Inhibitor
1 mol of quinoline was added into a four-necked reaction flask equipped with a stirrer, reflux condenser and thermometer, and then heated to 90˚C. 1 mol benzyl chloride was dropped into the quinoline solution and the mixture was heated to 140˚C and kept at that temperature for 2h. After the reaction, the solution was cooled down to the room temperature within 30 min. The precipitate was collected and compounded with 127.5 g OP-10 and 255 g ethanol after the solution was cooled down to room temperature naturally, and then the quinoline quaternary ammonium salt corrosion inhibitor was obtained, the first reaction mechanism is as follows:
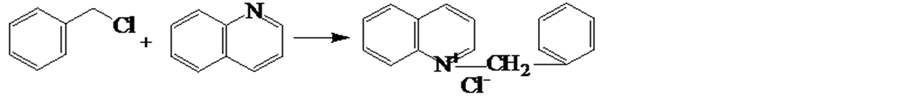
Epoxy chloropropane Quinoline Benzyl Quinoline
2.2.Results and Discussion
2.2.1.The Influencing Factors of Corrosion Inhibitor DNPerformance
In this test, theperformance of the corrosion inhibitor DN can be determined by the type and amount of initiator, the reaction mole ratio of quinoline and epoxy chloropropane and the reaction temperature, reaction time and other factors by the control. Thus, for the synthesis of carbon dioxide inhibitor must to study the impact of various factors on corrosion inhibition properties through repeated experiments, And ultimately determine the optimal synthesis conditions to obtain desirable properties of the product.
2.2.2.The Effect of the Reaction Mole Ratio of Epoxy Chloropropane and Quinoline
Greater effect of the reaction mole ratio of epoxy chloropropane and quinoline on the performance of corrosion inhibitor, in order to examine the effect of the reaction mole ratio on the performance of corrosion inhibitor, all the reaction conditions are not changed, the corrosion inhibition performance of corrosion inhibitorDN as shown in Table 1.
From the aboveTable 1, it can be seen thatwhen the reaction mole ratio of epoxy chloropropane and quinoline is 1:1, the corrosion rate is the lowest(0.1008mm/a) and the inhibition efficiency is the highest(97.5%), so determine the optimal ratio of 1:1.
2.2.3.The Effect of Reaction Time
Changes the reaction time have a great impact on the performance of corrosion inhibitor. In order to examine the effect of the time on the performance of corrosion inhibitor, all the other reaction conditions are not changed except reaction mole ratio of epoxy chloropropane and quinoline is 1:1, the performance of corrosion inhibitor DN as shown in Table 2.
From the aboveTable 2, it can be seen thatwhen the reaction time is 3 hours, the corrosion rate is the lowest(0.0953mm/a) and the inhibition efficiency is the highest(97.6%), the inhibition effect is the best, so determine the optimal reaction time is 3 hours.
2.2.4.The Effect of Reaction Temperature
Changes the reaction temperature have a great impact on the performance of corrosion inhibitor. In order to examine the effect of the reaction time on the performance of corrosion inhibitor, under conditions of 1:1 mole ratio of epoxy chloropropane and quinoline, the reaction time is 4 hours, changing the reaction temperature, the performance of corrosion inhibitor DN as shown in Table 3.
As can be seen from the Table 3, the reaction temperature is significantly influence the reaction rate and the degree of corrosion inhibitor, thus affecting the performance of the corrosion inhibitor. The synthesis temperature too low or too high are both not conducive to the reaction.When the reaction temperature is 130˚C, the corrosion rate is the lowest(0.1104mm/a) and the inhibition efficiency is the highest(95.1%), the inhibition effect is the best, so determine the optimal reaction temperature is 130˚C.
3. Evaluation of Corrosion Inhibitor Performance
3.1.TheEvaluation of Thermal Stability
According to the experimental method of thermal stability evaluation methods of corrosion inhibitor of oil field [2] , put three copies of corrosion inhibitor DN in different temperature and curing for 24 hours, the concentration of corrosion inhibitor is 1%, then observe what happened of this solution, the thermal stability of corrosion inhibitor DN as shown in Table 4.
As can be seen from the Table 4, under the different temperature 30˚C, 60˚C and 90˚C, the corrosion inhibitor
Table 1.The effect of reaction mole ratio of epoxy chloropropane and quinoline on the performance of corrosion inhibitor DN.
Table 2.The effect of time on the on the performance of corrosion inhibitor DN.
Table 3.The effect of reaction temperature on the performance of corrosion inhibitor DN.
Table 4. The evaluation of thermal stability of corrosion inhibitor DN.
solution has no stratification, no precipitation and the solution aqueous homogeneous, all these phenomenon show that the thermal stability of corrosion inhibitor DN is good.
3.2.TheEvaluation of Water Solubility
According to the experimental method of water solubility evaluation methods of corrosion inhibitor of oil field, put several copies of corrosion inhibitor of different concentrations in the thermostat, the concentrations of corrosion inhibitor solution are 200mg/L, 1000mg/L, 2000mg/L, 5000mg/L and 10,000mg/L, adjust the thermostat to 30˚C.After 30 minutes and 24 hours, observe what happened of this solution then note it[2] . The water solubility of corrosion inhibitor DN as shown inTable 5.
As can be seen from the Table 5, under 30˚Cand after 30 minutes and 24 hours, these five copies of corrosion inhibitor solution have no stratification, no precipitation and the solution aqueous homogeneous, the colour of the solution is dark red, all these phenomenon show that the corrosion inhibitor DN has good water solubility.
3.3.TheEvaluation of Compatibleness
In development course of oil field, the single corrosion inhibitor can not be used.The corrosion inhibitor always be used with all kinds of different treatment agent such as demulsifying agent, scale inhibitor [3] , bactericide and so on. If the corrosion inhibitor can take reaction with these treatment agents and give a precipitate or the other products, it can influencing the performance of the corrosion inhibitor directly, thus may affecting the development schedule of the oil field. Use the bactericide TQ-1 that usually used in oil field, scale inhibitor HEDP, clay stabilizer FL and demulsifying agent PC as the compatibility experiment. Measuring the scale inhibition rate by the methods that specified in the petroleum and natural gas industry standards SY/T5673. Measuring the bactericidal rate by the methods that specified in the petroleum and natural gas industry standards SY/T5890-93 and using the methods that specified in the petroleum and natural gas industry standards SY/T5273-2000 to measure the inhibition efficiency of corrosion inhibitor DN. The result of compatibleness as shown inTable 6.
As theTable 6shown, adding the different kinds of treatment agent in the corrosion inhibitor DN, the inhibition efficiency is better than ever before and various functions of the other treatment agent do not be changed. So the compatibleness of corrosion inhibitor DN with bactericide TQ-1, scale inhibitor HEDP, clay stabilizer FL and demulsifying agent PC is good.
4. Summary
1) Thecorrosion inhibitor DN is a kind of novel corrosion inhibitor that usesbenzyl chloride and quinoline as the raw material, synthesis a kind of quinoline quaternary ammonium salt, then makes the product benzyl quinoline compound with ethanol and OP-10.
Table 5.The evaluation of water solubility of corrosion inhibitor DN.
Table 6.The evaluation of compatibleness of corrosion inhibitor DN.
2)According to the large amounts of evaluation experiments of corrosion inhibitor performance, as the results shown, the thermal stability and water solubility of corrosion inhibitor DN are good and DN can compound with various treatment agents, enhancing the inhibition efficiency, the compatibleness of corrosion inhibitor DN is good.
Acknowledgements
The project is supported by PetroChina Innovation Foundationof Cationic Gemini Surfactant Property Evaluation and Oil Displacement Mechanism Study. Fund No.: 2013D-5006-0205
References
- Zhang, X.Y., Wang,F.P.,He,Y.F.andDu, Y.L.(2001) Study of the Inhibition Mechanism of Imidazoline Amide on CO2Corrosion of Armcoiron. Corrosion Science, 43, 1417-1431.http://dx.doi.org/10.1016/S0010-938X(00)00160-8
- Liang, L.(2010) Synthesis and Performance Evaluation of Quaternary Ammonium Salt Corrosion Inhibitor. Chemical Engineering, 38, 235-237.
- Crolet, J.L. (1994) Predicting CO2 Corrosion in Oil and Gas Industry. The Institute of Materials, London, 1.


