Open Journal of Inorganic Chemistry
Vol.2 No.2(2012), Article ID:18674,7 pages DOI:10.4236/ojic.2012.22006
Synthesis, characterization, spectroscopic and crystallographic investigation of Cobalt(III) schiff base complex with two perpendicular diamine coumarin ligands
![]()
1Unité de Recherche de Chimie des Matériaux et de l’Environnement, Université de Tunis El Manar, Tunis, Tunisia
2INSAT, Université de Carthage, Tunis, Tunisia
3Institute of Physics, Academy of Sciences of the Czech Republic, Praha, Czech Republic
Email: *rached.benhassen@fss.rnu.tn
Received 7 December 2011; revised 28 December 2011; accepted 16 January 2012
Keywords: Hydroxycoumarin Derivative; CoIII Complex; 1H NMR; UV-visible; Single Crystal X-Ray Diffraction
ABSTRACT
New transition metal complex of Cobalt(III) of the ligand (E)-3-(1-(2-aminoethylimino)ethyl)-4-hydroxy- 2H-chromen-2-one, derived from condensation of ethylene diamine with 3-acetyl-4-hydroxy-chromene- 2-one have been synthesized by reaction of cobalt(III) salt and the ligand, in amounts equal to metal-ligand molar ratio of 1:2. Both the Schiff base and the complex of Co(III) were characterized by IR, UV-vis, 1H NMRand 13C NMR-spectroscopy techniques. Single crystal X-ray diffraction investigation, at low temperature T = 120 K, shows that the cobalt complex is triclinic P-1, a = 10.426(5) Å, b = 11.3234(2) Å, c = 15.729(5) Å, α(˚) = 70.102(4), β(˚) = 86.049(4), γ(˚) = 82.497(4), Z = 2, and its structure consists of isolated [Co(III)(C13H13N2O3)2]+ complex cations with distorted octahedral geometry,  counter anions, acetone solvent and water molecules. The crystal cohesion is stabilized by hydrogen bonds between ligands and water molecules, and ionic interactions between complex cations and counter anions.
counter anions, acetone solvent and water molecules. The crystal cohesion is stabilized by hydrogen bonds between ligands and water molecules, and ionic interactions between complex cations and counter anions.
1. INTRODUCTION
Coumarins and their derivatives [1,2] were found to exhibit a variety of biological and pharmacological activeties and have attracted considerable interest because of their potential beneficial effects on human health [3,4]. A series of coumarin derivatives were recently synthesized and studied for their structure and anticoagulant activity and cytotoxic [5-10]. A number of coumarins have been investigated for complexing ability [11,12]. The mechanisms of cytotoxic action of a large number of different coordination compounds have been discussed in relation to the development of new antitumor agents.
A wide range of medicinal applications of metal complexes of coumarins was investigated. It was found that in some cases, the metal complexes obtained revealed a higher activity than their biological ligands [9-11].
In the past decade, Schiff bases have received much attention, mainly because of their extensive applications in the synthesis of coordination compounds and catalysis [13-17]. This is due to the fact that Schiff bases offer opportunities for inducing substrate chirality, tuning factors of metal centered electronic and improve the solubility and stability of homogeneous or heterogeneous catalysts, i.e. [18,19]. They are also increasingly important as biochemical, analytical and antimicrobial reagents [20]. Further, significant advances have also been achieved in the field of straightforward preparation of new chiral ligands, such as chiral diamines and complexes, used for asymmetric catalysis [21]. For example, complexes based on chiral diamines have been employed for chiral hydrogenations and transfer hydrogenations [22]. Both homogeneous and heterogeneous diamine complexes have proved highly effective in enantioselective catalysis [23,24].
In continuation of our previous research [25-27] on synthesis and characterization of new asymmetric Schiff base complex, we describe here the synthesis and characterization of the tridentate Schiff base (E)-3-(1-(2- aminoethylimino)ethyl)-4-hydroxy-2H-chromen-2-one (1), then its complexation reaction with cobalt(III) to obtain the complex numbered as (2).

2. EXPERIMENTAL
2.1. Measurements
Proton nuclear magnetic resonance (1HNMR) spectra were run on a Bruker AV-300 NMR spectrometer and chemical shifts expressed as d (parts per million) values with TMS as internal standard. Multiplicities of proton resonance are designated as singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). IR spectra were recorded in KBr on a Bruker FT-IR spectrophotometer. The electronic spectra in the 200 - 900 nm range were obtained on a Varian spectrophotometer 50 scan, using solutions with the concentration of 10–5 M in DMSO. Single crystal was characterized by an Oxford Diffraction Gemini R Ultra CCD diffractometer. All the chemicals are commercially available and they were used without further purification. All the solvents were dried using standard methods before use.
2.2. Synthesis of the Schiff Base (1)
The reaction of 3-acetyl-4-hydroxy-2H-chromen-2-one with the ethylene diamine in the presence of methanol provided a high yield, as detailed below.
A solution of (0.6 mL, 5 mmol) ethylene diamine in methanol was added drop wise stirring to a methanol solution of (2 g, 5 mmol) 3-acetyl-4-hydroxy-chromene- 2-one, already prepared by the method cited in [28], and then the mixture was refluxed for 1 h. The precipitated Schiff base was filtered and recrystallized from DMSO. Yield: 1.7 g (85%).
IR (selected, cm–1): 3097 [n(C-H arom)], 3207 [n(NH2)], 1637 [n(C=O)], 1618 [n(C=N)], 1585 [n(C=C)], 1279 [n(C-O)].
UV-vis λmax/nm (DMSO): 235, 250, 320.
1HNMR (300 MHz, DMSO): δ 13.76 (s, H, OH), 7.23 - 8.27 (m, 4H, H arom), 4.00 (s, 2H, NH2), 2.69 (d, 2H, CH2), 2.48 (t, 2H, CH2), 2.07 (s, 3H, CH3).
13CNMR (75MHz, DMSO): δ 179.63 C(21), 177.20 C(10), 161.80 C(8), 152.99 C(6), 134.12 C(2), 125.63 C(4), 123.68 C(3), 120.04 C(1), 116.20 C(9), 96.47 C(5), 42.81 C(25), 34.40 C(24),18.31 C(52).
2.3. Synthesis of the Complex [Co(C13N2O3H13)2]ClO4, 2(C3H6O), H2O (2)
In the synthesis of (2), one equivalent of ethylene diamine condenses with 4-hydroxy-2H-chromen-2-one to give 1:1 Schiff base (1) that is sequestered as, CoIII complex (2) (figure 1).
A solution of (0.34 g, 1 mmol) of Co(C2H3O2)2·4H2O dissolved in 50 mL methanol was added to the freshly prepared Schiff base (0.41 g, 2 mmol). Upon addition of the metal, the solution immediately turned brown. An aqueous solution of sodium perchlorate to this solution was added and then the color turned red. The mixture was stirred for 3 h and the solid was collected by gravity filtration and dried under vacuum. Yield: 70%.
IR (selected, cm–1): 3414 [ν(OH)], 1602 [ν(C=N)], 1418 [ν(C-O)], 1084.19 [ν(Cl-O) (ClO4 )], 523 [ν(M_N)], 418 [ν(M_O)].
UV-vis λmax/nm (DMSO): 552, 486, 346, 261.
1H_NMR: (300MHz, DMSO): δ 7.19 - 7.8 (m, 4H, H-arom.), 3.99 (t, 2H, CH2), 2.90 (s, 3H, CH3), 2.49 (d, H, NH), 2.48 (d, 2H, CH2).
13C NMR (75 MHz, DMSO): δ 172.56 (C10), 161.85 (C21), 152.8 (C8), 133.42 (C4), 125.92 (C2), 123.63 (C4), 119.38 (C1), 115.56 (C5), 100.83 (C9), 55.54 (C25), 42.94 (C24), 22.35 (C52), 119.38 (C3).
2.4. X-Ray Data Collection and Structure Solution and Refinement
Single crystals, suitable for X-ray data collection, were obtained by slow evaporation of the solvent from a solution of (2) in CH3COCH3. The crystals were filtered and washed with acetone. The diffraction data were collected with an Oxford Diffraction Gemini R Ultra CCD. Semiempirical absorption corrections from equivalents (multiscan) were applied, using graphite-monochromated Cu Kα

Figure 1. The synthesis steps of the title compound.
radiation at 120 K. The structure was solved with direct methods using SIR 2004 [29]. An anisotropic refinement, by full-matrix least-squares was performed with JANA2006 [30], and the residual finally converged to R1 = 0.08. Non-hydrogen atoms were anisotropic and the hydrogen atom positions were included in the riding mode, except for those of water molecule, where they are introduced and refined with distance and angle restrictions. Acetone solvent molecules are not coordinated to cobalt. They just, fill voids in the crystal lattice. One of the two acetone molecules is quite involved in disorder and so, we let it to possess two positions in the same void. For this purpose, a split model in which two slightly different positions for the atoms C45, C44, O46 and C47 was used. Both refined positions had different occupancy and their sum was significantly different from 1. The disorder of this solvent molecule has negative influences on the structure because of the relatively high number of electrons that are disordered with the solvent. This, together with the very weak diffractions, meant that the R values are higher than usual, but the molecular structure is clear. Further details are given in Table 1. Selected bond lengths and angles are listed in Table 2" target="_self"> Table 2. The structure exhibits intermolecular hydrogen bonds (Table 3).
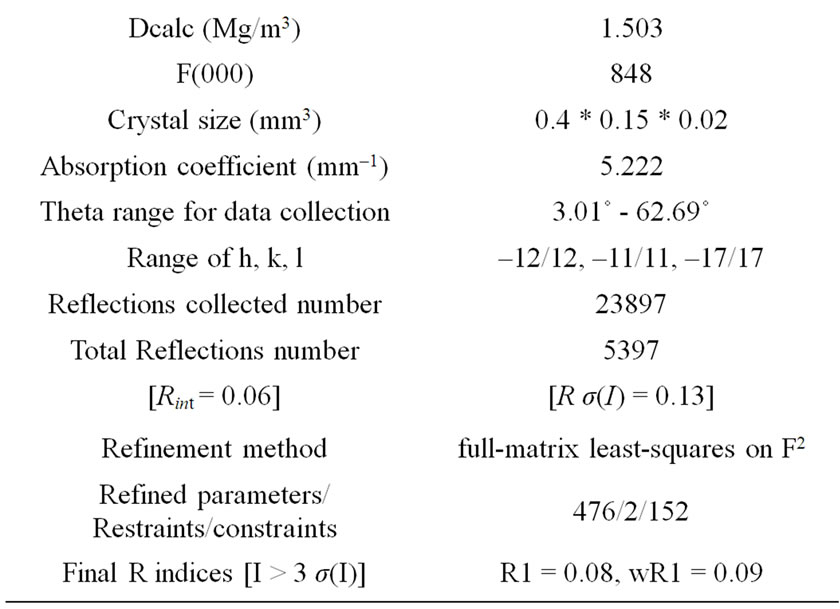

Table 1. Crystal data and structure refinement for cobalt complex.

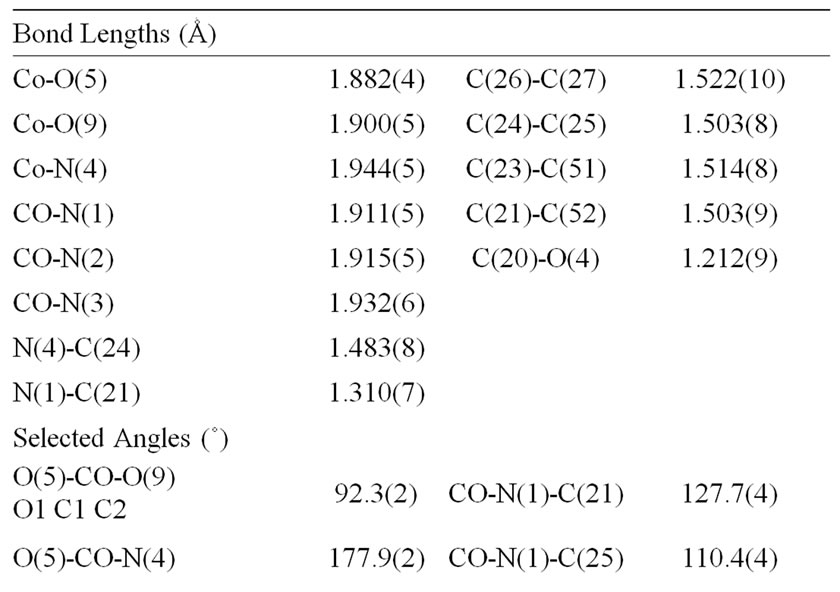
Table 2. Selected bond distances (Ǻ) and angles (˚).

Table 3. Hydrogen bonds for the title compound (Ǻ and ˚).
3. RESULTS AND DISCUSSIONS
The Schiff base was isolated in 85% yield and was obtained cleanly, melting point 170˚C, yellow in color, insoluble in common organic solvents, partially soluble in chloroform but soluble in dimethyl sulfoxide (DMSO). The reaction of the ligand and metal salt gave the corresponding metal complex in good yield, stable and nonhygroscopic in nature. The complex was prepared red in color and was found to be soluble in DMSO, CH3COCH3 but was only partially soluble in other organic solvents such as CHCl3 and CH3OH. It decomposes at about 322˚C.
3.1. Spectral Characterization of the Ligand and Complex
The IR, 1H NMR and 13C NMR spectra of the synthesized materials confirm the formation of ligand and complex with the proposed structure of the type [Co(C13N2O3H13)2]ClO4, 2(C3H6O), H2O.
3.2. IR Spectrum
The IR spectrum of the Schiff base exhibits characteristic intensity band at 1618 cm–1 which is attributed to the ν(C=N) vibration. Another band at 1637 cm–1 is assigned to ν(C=O), lactonyl carbon of the coumarin moiety. The Schiff base has a strong absorption band at 3207 cm–1, which corresponds to the NH2 stretching frequency [31]. A medium intensity band at 1585 cm–1 is regarded as an aromatic C=C stretching vibrations.
In comparison with the spectrum of the Schiff base, the complex exhibits the band of ν(C=N) in the region 1602 cm–1, showing the shift of band to lower wave numbers indicating that, the azomethine nitrogen is coordinated to the metal ion. The high intensity band due to enolic C-O appeared at 1325 cm–1 in the Schiff base, appeared as a medium to high intensity band in the 1418 cm–1 region in the complex.
These observations support the formation of M-O bonds via deprotonation. The broad band of medium intensity occurring in the 3414 cm–1 region, corresponds to of water molecules.
The bands in the region of 410 - 375 and 524 - 480 cm–1 in the complex are assigned to stretching frequencies of (M-O) and (M-N) bonds respectively. The absorption bands near 523 cm–1 and 418 cm–1 are related to the coordination of Schiff-base to cobalt ion through Co-O and Co-N bands [32].
3.3. Electronic Spectrum
The electronic spectrum (Figure 2) of the free Schiff base ligand shows absorption bands in the ranges 215 - 260 and 270 - 367 nm. The absorption maxima at 235 nm and 250 nm are due to π-π∗ transitions for the benzene ring and enol ring respectively. That observed at 320 nm is reasonably assigned to π-π∗ transition for azomethine moiety. The shoulder at 338 nm corresponds to n-π∗ transitions for azomethine and NH2 groups [33]. In the spectrum of the complex (Figure 2), the absorption bands at ca. 261, 320, 346 nm of the enol, azomethine and NH2 chromophores π-π∗ and n-π∗ transitions are shifted compared to the ligand indicating that the enolic oxygen, the imine and amine nitrogen are involved in the coordination with metal ion.
The spectrum of the metal complex shows (inset of Figure 2) that the absorption around 400 - 600 nm is due to ligand to metal charge transfer and d-d* transition band of the metal in the complex.

Figure 2. UV-vis absorption spectra of ligand (red spectrum) and complex (blue spectrum). The inset shows the vis. Spectrum of the complex in the region 400 - 600 nm.
3.4. NMR Spectra
The Schiff base and the complex has been characterized by 1H and 13C NMR spectra.
In 1H NMR spectrum of the Schiff base, signal at 13.76 is ascribed to enolic-OH and the methyl protons from the acetyl fragment are identified at 2.07 ppm. The 1H-NMR spectrum of the Schiff base showed the characteristic coumarin aromatic proton signals in the 7.23 - 8.27 ppm. Two additional signals at 2.48 ppm and 2.69 ppm are ascribed to CH2-CH2. The signal around 4.00 ppm is ascribed to NH2.
The comparison of the 1H-NMR spectra of the complex and ligand leads to: The resonance due to enolic-OH, around 13.76 ppm in the Schiff base disappears in the spectrum of complex; this confirms that, the hydroxyl group reacted with metal ion via deprotonation.
In 13C NMR spectra of the Schiff base, the signals appeared in the region 116.20 - 152.99 ppm are assigned to aromatic carbons. The signals at 179.63, 161.80, 18.31 ppm are due to C=N, C=O and CH3 respectively. Additional signals at 34.40 ppm and 42.81 ppm are ascribed to CH2-CH2.
The comparison of the 13C NMR spectra of the complex and ligand leads to: The azomethine (C=N) carbon signal occurring, as single at 179.63 ppm in the ligand, moves upfield to 161.85 upon complexation. This provides further support for the coordination of the ligand through the azomethine nitrogens to metal ions.
3.5. X-Ray Structure Determination of Complex Cobalt
A relatively high coordination number of 6 is achieved by Co, involving two tridentate Schiff base ligands, as shown in Figure 3 The molecular structure of the complex consists of discrete [Co(C13H13N2O3)2]+ cation,  counter anion, acetone solvent and water molecules.
counter anion, acetone solvent and water molecules.
Figure 4 Shows the packing of the structure. The cobalt(III) center has a distorted octahedral environment,
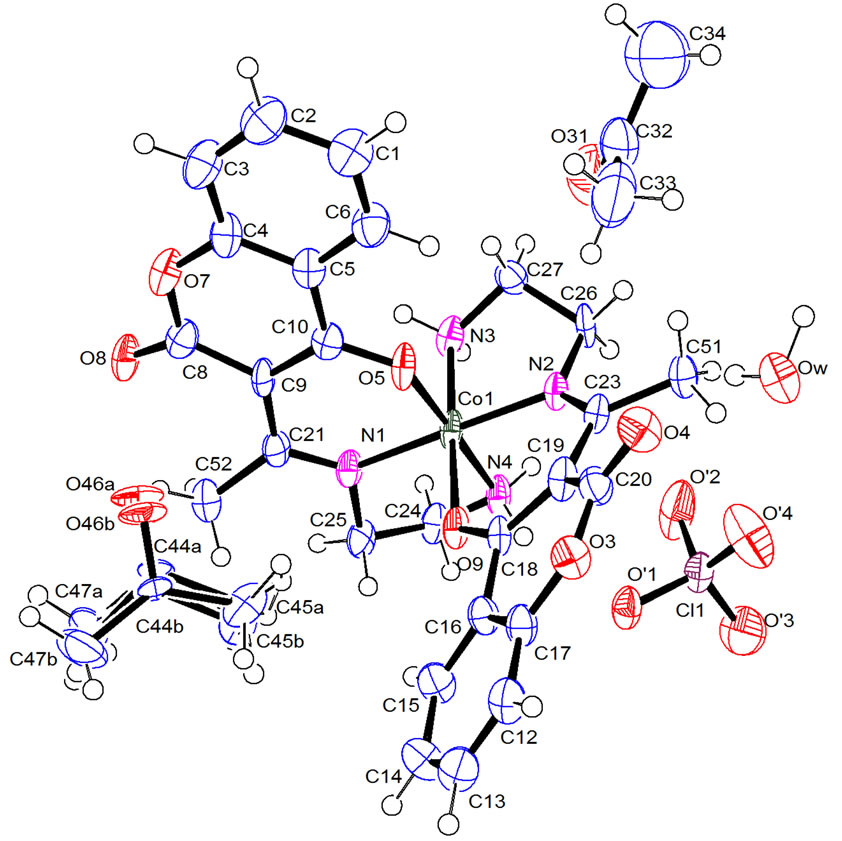
Figure 3. ORTEP stereoscopic projection of the molecular structure of [Co(L)2]ClO4, 2(C3H6O), H2O. Thermal ellipsoids are given at 50% probability.

Figure 4. The packing of the structure.
with four nitrogen atoms and two oxygen atoms of the two perpendiculars tridentate ligands.
The complex coordinates through imido nitrogen and enolic oxygen with both the nitrogen donors and the enolic oxygen donors being cis. The Co-O [1.882(4) Å and 1.944(5) Å] bond lengths are in the range of those expected for CoIII in an octahedral environment [34]. The distortion, from regular octahedral geometry is evident from the N(1)-Co-N(2) bond angle of 179.2(2)˚, from O(5)-Co-N(3) and O(9)-Co-N(4) where the angles are 90.3(2)˚ and 85.6(2)˚ respectively. The calculated average values of the distortion indices [34,35], corresponding to the different angles and distances in the octahedron, when assuming that the Co-O and Co-N bonds are identical, [DI((O/N)Co(O/N)) = 0.003, DI(Co(O/N)) = 0.011, DI((O/N)(O/N)) = 0.055] exhibit a pronounced distortion of the Co(O/N) distances, if compared to (O/N)Co(O/N) angles and (O/N)(O/N) distances.
The diamino fragment is twisted at a torsion angle of 13.3(1)˚ from the fused ring plane. The dimensions in the shift base correspond closely to standard values [36] except for the bonds C9-C8, C9-C10 and C10-C5, where C9-C10, a double bond, is longer (at 1.414(9) Ǻ) than the expected 1.340Ǻ, C10-C5 is longer (at 1.447(10) Ǻ) than the expected 1.440 Ǻ, and C9-C8 is shorter (at 1.456(9) Ǻ) than the expected 1.465 Ǻ.
The bond angles C3-C4-O7 and C6-C5-C10 at the junction of the two rings are 117.6(6)˚ and 123.6(6)˚, respectively. Here, as well as in other coumarin compounds [37], an important asymmetry in the O-C-O bond angles was detected; O7-C8-O8=114.8(6)˚.
The crystal structure cohesion is stabilized by N---H···O hydrogen bonds between neighboring ligands, and O---H···O links between water molecules and the ligands on the one hand and counter-anions on the other, and ionic interacttions between complex cations and counter-anions, these hydrogen bonds link the molecules into a three-dimensional frame. In the O---H···O hydrogen bonds, water mole-cules were proton donors. Strong H-bonding was observed when the lactone group was a potential hydrogen acceptor (see Table 3 for the hydrogen bond [OwHw1···O4]).
4. CONCLUSION
The newly synthesized Schiff base which acts as a tridentate ligand was derived in good yield from condensation of ethylene diamine with 3-acetyl-4-hydroxychromene-2-one. It coordinated with the metal ion with two azomethine nitrogen and enolic oxygen atom by deprotonation. The bonding of ligand to metal ion is confirmed by spectroscopic investigation. The structure of [Co(L)2]+ was determined by single crystal X-ray diffraction and revealed that the NNO-donor sets of two ligand fragments form a distorted octahedral configuretion at the Cobalt ion. A perpendicularity in the arrangement of the two Schiff base ligands is observed. The structure of the complex revealed the existence of network in one-dimensional zigzag chain propagating along the c axis in which the perchlorate ions, the acetone solvent and water molecules are inserted between the ab planes.
5. SUPPLEMENTARY MATERIAL
CCDC 840711 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
REFERENCES
- Galm, U., Heller, S., Shapiro, S., Page, M., Li, S. and Heide, L. (2004) Antimicrobial and DNA gyrase-inhibitory activities of novel clorobiocin derivatives produced by mutasynthesis. Antimicrobial agents and chemotherapy, 48, 1307-1312. doi:10.1128/AAC.48.4.1307-1312.2004
- Tao, J., Hu, S., Pacholec, M. and Walsh, C.T. (2003) Synthesis of proposed oxidation-cyclization-methylation intermidiates of the coumarin antibiotic biosynthetic pathway. Organic Letters, 5, 3233-3236. doi:10.1021/ol035101r
- O’Kennedy, R. and Thornes, R.D. (1997) Coumarins: biology, applications and mode of action. John Wiley & Sons, New York.
- Hoult, J.R. and Paya, M. (1996) Pharmacological and biochemical actions of simple coumarins natural products with therapeutic potential. General Pharmacology, 27, 713-722. doi:10.1016/0306-3623(95)02112-4
- Kam, C.M., Kerrigan, J.E., Plaskon, R.R., Duffy, E.J., Lollar, P., Suddath, F.L. and Powers, J.C. (1994) Mechanism-based isocoumarin inhibitors for blood coagulation serine proteases. effect of the 7-substituent in 7-amino-4- chloro-3-(isothioureidoalkoxy)isocoumarins on inhibitory and anticoagulant potency. Journal of Medicinal Chemistry, 37, 1298-1306.
- Lakin, K.M., Smirnova, T.V., Vishnyakova, G.M., Lobanova, E.G., Novikova, N.V. and Sklyarenko, V.I. (1989) New water-soluble anticoagulants of the coumarin series. Pharmaceutical Chemistry Journal, 23, 824-826.
- Finn, G.J., Creaven, B. and Egan, D.A. (2001) Study of the in vitro cytotoxic potential of natural and synthetic coumarin derivatives using human normal and neoplastic skin cell lines. Melanoma Research, 11, 461-467. doi:10.1097/00008390-200110000-00004
- Kerr, J.S., Li, H.Y., Wexler, R.S., Robinson, A.J., Robinson, C.S., Boswell, G.A., Kranthanser, C. and Harlow, P.P. (1997) The characterization of potent novel warfarin analogs. Thrombosis Research, 88, 127-136. doi:10.1016/S0049-3848(97)00224-7
- Ammar, H.O., Ghorab, M., El Nahhal, S.A. and Makram, T.S. (1997) Interaction of oral anticoagulants with methyl xanthines. Pharmazie, 52, 946-950.
- Kostova, I. (2005) Antineoplastic activity of new lanthanide (cerium, lanthanum and neodymium) complex compounds. Current Medicinal Chemistry—Anti-Cancer Agents, 5, 29-46. doi:10.2174/1568011053352550
- Mandakmare, A.U. and Navwade, M.L. (1997) Stability constants of Fe(III), Cr(III), Al(III) chelates with some substituted coumarins. Oriental Journal of Chemistry, 13, 155-158.
- El-Ansary, A.L. and Omar, N.M.M. (1988) New lanthanide complexes of 4-methyl-7-hydroxycoumarin and their pharmacological activity. Egyptian Journal of Chemistry, 31, 511-520.
- Goswami, N. and Eichhorn, D.M. (2000) Incorporation of thiolate donation using 2,2’-dithiodibenzaldehyde: synthesis of Ni, Fe, and Cu complexes. Crystal structures of [M(tsalen)] (tsalen = N,N’-ethylenebis(thiosalicylidene)- imine); M = Cu, Ni) showing a tetrahedral distortion of Cu(tsalen). Inorganica Chimica Acta, 303, 271-276. doi:10.1016/S0020-1693(00)00047-5
- Labisbal, E., Rodriguez, L., Sousa-Pedrares, A., Alonso, M., Vizoso, A., Romero, J., Garcia-Vazquez, J.A. and Sousa, A. (2006) Synthesis, characterisation and X-ray structures of diorganotin(IV) and iron(III) complexes of dianionic terdentate Schiff base ligands. Journal of Organometallic Chemistry, 691, 1321-1332. doi:10.1016/j.jorganchem.2005.09.052
- Yu, T.z., et al. (2006) Synthesis, crystal structure and electroluminescent properties of a Schiff base zinc complex. Inorganica Chimica Acta, 359, 2246-2251. doi:10.1016/j.ica.2006.01.019
- Hoshina, G., Tsuchimoto, M., Ohba, S., Nakajima, K., Uekusa, H., Ohashi, Y., Ishina, H. and Kojima, M. (1998) European Journal of Inorganic Chemistry, 37, 142-147. doi:10.1021/ic9705958
- Canali, L. and Sherrington, D.C. (1999) Utilisation of homogeneous and supported chiral metal (salen) complexes in asymmetric catalysis. Chemical Society Reviews, 28, 85-93. doi:10.1039/a806483k
- Opstal, T. and Verpoort, F. (2003) Synthesis of highly active ruthenium indenylidene complexes for atom-transfer radical polymerization and ring-opening-metathesis polymerization. Angewandte Chemie International Edition, 42, 2876-2879. doi:10.1002/anie.200250840
- Clercq, B.D., Lefebvre, F. and Verpoort, F. (2003) Immobilization of multifunctional Schiff base containing ruthenium complexes on MCM-41. Applied Catalysis A, 247, 345-364. doi:10.1016/S0926-860X(03)00126-1
- Tumer, M., Koksal, H., Sener, M.K. and Serin, S. (1999) Antimicrobial activity studies of the binuclear metal complexes derived from tridentate Schiff base ligands. Transition Metal Chemistry, 24, 414-420. doi:10.1023/A:1006973823926
- Desimoni, G., Faita, G. and Jorgensen, K.A. (2006) C(2)- symmetric chiral bis(oxazoline) ligands in asymmetric catalysis. Chemical Reviews, 106, 3561-3651. doi:10.1021/cr0505324
- Fache, F., Schulz, E., Tommasino, M.L. and Lemaire, M. (2000) Nitrogencontaining ligands for asymmetric homogeneous and heterogeneous catalysis. Chemical Reviews, 100, 2159-2232. doi:10.1021/cr9902897
- Thomas, J.M. and Raja, R.J. (2004) Catalytic significance of organometallic compounds immobilized on mesoporous silica: economically and environmentally important examples. Journal of Organometallic Chemistry, 689, 4110-4124. doi:10.1016/j.jorganchem.2004.07.052
- Jones, M.D. and Mahon, M.F. (2008) Synthesis of Rh(I) diamine complexes and their exploitation for asymmetric hydrogen transfer processes. Journal of Organometallic Chemistry, 693, 2377-2382. doi:10.1016/j.jorganchem.2008.04.020
- Dreos, R., Mechi, L., Randaccio, L., Siega, P., Zangrand, E. and Ben Hassen, R. (2006) Synthesis and X-ray crystal structure of a new μ-hydroxo dinuclear cobalt complex containing one cis-β folded and one planar salen moiety. Journal of Organometallic Chemistry, 691, 3305-3309. doi:10.1016/j.jorganchem.2006.04.006
- Dreos, R., Mechi, L., Nardin, G., Randaccio, L. and Siega, P. (2005) Alternative cocrystallization of “almost” enantiomers and true enantiomers in some cis-β-organocobalt salen-type complexes with α-amino acids. Journal of Organometallic Chemistry, 690, 3815-3821. doi:10.1016/j.jorganchem.2005.05.017
- Mechi, L., Chtiba, S., Hamdi, N. and Ben Hassen, R. (2009) 4-Hydr-oxy-3-[(2E)-3-(3,4,5-trimethoxy-phen-yl)- prop-2-eno-yl]-2H-chromen-2-one. Acta Crystallographica Section E, 65, 1652-1653. doi:10.1107/S1600536809022569
- Dholakia, V.N., Parekh, M.G. and Trivedi, K.N. (1968) Studies in 4-hydroxy coumarins. Australian Journal of Chemistry, 21, 2345-2347. doi:10.1071/CH9682345
- Burla, M.C., Caliandro, R., Camalli, M., Carrozzini, B., Cascarano, G.L., De Caro, L., Giacovazzo, C., Polidori, G. and Spagna, R. (2005) SIR2004: An improved tool for crystal structure determination and refinement. Journal of Applied Crystallography, 38, 381-388. doi:10.1107/S002188980403225X
- Petricek, V., Dusek, M. and Palatinus, L. (2006) Jana 2006. Institute of Physics, Praha.
- Bellamy, L.J. (1975) The infra-red spectra of complex molecules. 3rd Edition, Chapman and Hall, London.
- Bhattacharjee, S. and Anderson, J. (2006) Comparison of the epoxidation of cyclohexene, dicyclopentadiene and 1,5-cyclooctadiene over LDH hosted Fe and Mn sulfonato-salen complexes. Journal of Molecular Catalysis A, 249, 103-110. doi:10.1016/j.molcata.2005.12.042
- Zhang, Y., Zhao, J., He, L., Zhao, D. and Zhang, S. (2006) Manganese(III) salen complex anchored onto MCM-41 as catalyst for the aerobic epoxidation of olefins. Microporous and Mesoporous Materials, 94, 159-165. doi:10.1016/j.micromeso.2006.03.040
- Ertl, A., Huches, J.M., Pertlik, F., Foit, F., wright, S.E., Brandstatter, F. and Marler, B. (2002) Polyhedrom distrtions in tourmaline. The Canadian Mineralogist, 40, 153- 163. doi:10.2113/gscanmin.40.1.153
- Baur, W.H. (1974) the geometry of polyhedral distortions predictive relationships for the phosphate group. Acta Crystallographica, 30, 1195-1215.
- Gupta, S.K., Hitchcock, P.B. and Kushwah, Y.S. (2002) The crystal structure of 4-methyl-2,6-dibenzoylphenol and its conversion into a mononuclear cobalt(III) complex by treatment with cobalt(II) chloride and propane- 1,3-diamine. Polyhedron, 21, 1787-1793. doi:10.1016/S0277-5387(02)01044-6
- Traven, V.F., Manaev, A.V., Safronova, O.B., Chibisova, T.A., Lyssenko, K.A. and Antipin, M.Y. (2000) Electronic structure of p systems: XVIII.1 photoelectron spectrum and crystal structure of 3-acetyl-4-hydroxycoumarin. Russian Journal of General Chemistry, 70, 798-808.
NOTES
*Corresponding author.

