Advances in Nanoparticles
Vol. 2 No. 4 (2013) , Article ID: 38357 , 7 pages DOI:10.4236/anp.2013.24040
The Structure of Modified Fe-Ni Bioxide Composite Nanoparticles Using Fe(NO3)3
1School of Physical Science & Technology, Southwest University, Chongqing, China
2School of Mechanical and Control Engineering, Beijing Jiaotong University, Beijing, China
Email: *aizhong@swu.edu.cn
Copyright © 2013 Yueqiang Lin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 27, 2013; revised August 26, 2013; accepted September 11, 2013
Keywords: Nanoparticles; Composite; Treatment; Characterization
ABSTRACT
Composite nanoparticles containing a γ-Fe2O3 core, Ni2O3 external shell and FeCl3∙6H2O outermost layer can be synthesized by chemically induced transition in FeCl2 solution. These may be modified by treatment with Fe(NO3)3 to obtain particles for the preparation of ionic ferrofluids. Vibrating sample magnetometer (VSM) measurements and transmission electron microscopy (TEM) observations show that after Fe(NO3)3 treatment, the specific magnetization becomes weaker and the size becomes larger for treated particles compared with the untreated particles. Using energy dispersive X-ray spectroscopy (EDX), X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS), the structure of the particles before and after the treatment is revealed. The experimental results show that the γ-Fe2O3 core is unchanged, the Ni2O3 is dissolved partially and the FeCl3·6H2O is replaced by Fe(NO3)3∙9H2O. The percentages of molar, mass and volume of these phases are deduced, and the average density of the modified particles is also estimated.
1. Introduction
Magnetic nanoparticles are an important class of nanoscopic magnetic systems. The preparation and characterization of magnetic nanoparticles have attracted increasing interest as particles in this size range may allow investigation of fundamental aspects of magnetic-ordering phenomena in magnetic materials with reduced dimension and could lead to new technological applications [1,2]. A nanocomposite is a material composed of two or more phases in which the combination of different physical or chemical properties may lead to completely novel materials [3]. In addition, magnetic nanocomposites have applications that range from ferrofluids to separation science and technology [4].
Ferrofluids (magnetic fluids) can be synthesized by dispersing nanosized subdomain magnetic particles of ~10 nm in diameter in a carrier liquid. Generally, magnetic nanoparticles in ferrofluids are coated with a surfactant to prevent aggregation [5]. In the 1980s, Massart proposed a method for chemical synthesis of ferrofluids with no surfactant [6]. This method has subsequently been called the Massart’s method and such ferrofluids are known as ionic ferrofluids or electrical double-layered ferrofluids [7,8]. In an adaptation of Massart’s method for the synthesis of the ferrofluids, magnetic nanoparticles need to be treated with ferric nitrate to yield a chemically stable particle surface.
Magnetization (moment per unit volume) M is an important physical parameter used to characterize magnetic materials. In practice, the volume of a particle Vp is too difficult to measure directly, so the magnetization is obtained usually from M = s∙ρ, where s is the specific magnetization (moment per unit mass) and ρ is the known density of the constituent materials [9]. In addition, the volume fraction of particles in ferrofluids, ϕv = Vp/(Vp + Vc), where Vp is the volume of the particles and Vc is the volume of the carrier liquid, is also an important characteristic parameter to which the behavior of ferrofluids is related [10]. Therefore, volume of the particles is an important feature for magnetic nanoparticles and is generally obtained from an accurate measure of the mass m and the known density ρ, i.e. Vp = m/ρ. However, if the nanoparticles consist of different chemical compounds, the density of the particles is no longer uniform, and their volume cannot be determined by measuring just their mass.
Recently, we have proposed a method to prepare Fe-Ni bioxide composite nanoparticles by a chemically induced transition [11]. These nanoparticles might be very suitable for the synthesis of ionic ferrofluids [12]. In the present work, untreated Fe-Ni bioxide composite nanoparticles and those treated with ferric nitrate have been characterized, and their chemical compositions and average density were studied.
2. Experimental
The γ-Fe2O3/Ni2O3 composite nanoparticles were prepared by the so-called chemically induced transition method. The preparation can be divided into two steps. Firstly, an aqueous mixture of FeCl3 (40 ml, 1 M) and Ni(NO3)3 (10 ml, 2 M; in HCl 0.05 ml) was added to NaOH solution (500 ml, 0.7 M). Then the solution was heated and boiled for 5 min, after which the black precursor gradually precipitated. Secondly, the precursor was added to FeCl2 solution (400 ml, 0.25 M), which was then heated to boiling point for 30 min. The nanoparticles were again precipitated after the heating had stopped. After washing to pH = 7 with very dilute aqueous HNO3 solution (≤0.01 M), the as-prepared particles (the untreated particles) were added to boiling Fe(NO3)3 solution (400 ml, 0.25 M), which was then kept boiling for 30 min. The particles were then dehydrated with acetone and allowed to dry naturally to yield the modified particles (the treated particles).
The magnetization, morphology, bulk chemical species, crystal structure and surface chemical compositions of the particles were investigated using a vibrating sample magnetometer (VSM, HH-15), transmission electron microscopy (TEM, TecnaiG220), energy dispersive Xray spectroscopy (EDX, Quanta-200), X-ray diffraction (XRD, XD-12) and X-ray photoelectron spectroscopy (XPS, SAM800). The phase structures of the particles were determined from the experimental results.
3. Results and Analysis
The specific magnetization curves are shown in Figure 1. Clearly, the magnetization of the treated particles is weaker than that of the untreated particles. From the plots of s vs. 1/H in high field [13] the specific saturation magnetizations ss are evaluated as 56.59 emu/g and 41.19 emu/g for the untreated and treated particles, respectively.
TEM observations show that both untreated and treated particles were spherical. Figure 2 is a typical TEM micrograph of the particles. Statistical analysis reveals that the size of the particles fits a log-normal distribution. For the untreated particles, the median diameter

Figure 1. The specific magnetization curves of (a) untreated particles and (b) treated particles.


Figure 2. Typical TEM images of (a) untreated particles and (b) treated particles.
dg is 10.78 nm and the standard deviation lnsg is 0.27; for the treated particles, dg is 11.43 nm and lnsg is 0.26. The average diameter
The results of the EDX measurements indicated that both untreated and treated particles had O, Fe and Ni species, but the untreated particles also contained Cl and the treated particles contained N and Cl. The atomic percentages ai for these elements are listed in Table 1.
Figure 3 shows the XRD patterns for the two nanoparticles. For the untreated particles, the major diffraction peaks corresponded to γ-Fe2O3 (magnetite, PDF# 39-1346), with some weaker peaks corresponding to Ni2O3 (PDF#14-0481). No clear diffraction peaks corresponded to any phase based on the Cl species. In addition, for the treated particles, diffraction peaks were present for both γ-Fe2O3 and Ni2O3. However, by comparing with the pattern of untreated particles, it was found that the diffraction intensity of Ni2O3 had been weakened relative to that of γ-Fe2O3. In addition, some new diffraction peaks appeared, as indicated by arrows A, B, C and D. These are close to the d=0.2620, 0.2550, 0.1900 and 0.1700 nm peaks of Fe(NO3)3·9H2O (PDF#01-0124).

Figure 3. XRD patterns of (a) untreated particles and (b) treated particles. The peaks (h k l) correspond to γ-Fe2O3 (PDF#39-1346) and peaks (h k l)* correspond to Ni2O3 (PDF#14-0481).

Table 1. The atomic composition from EDX measurement ai.
The XPS spectra are shown in Figure 4. These confirm the presence of the same chemical species as determined by the EDX measurements. Combining the EDX and XRD results, it was apparent that besides γ-Fe2O3 and Ni2O3, there was also a phase based on Cl in the untreated particles and Fe(NO3)3·9H2O was present in the treated particles. As a result, the O1s and Fe2p3/2 lines are split, as shown in Figure 5. The detailed binding energy data are listed in Table 2. To summarize, these measurements precisely identify γ-Fe2O3, Ni2O3 and FeCl3 phases in the untreated particles and γ-Fe2O3, Ni2O3 and Fe(NO3)3∙9H2O phases in treated particles. In the untreated particles, the FeCl3 phase could contain

Figure 4. XPS spectra of (a) untreated particles and (b) treated particles.

Table 2. The binding energy data from XPS measurements (eV).
 (a)
(a) (b)
(b)
Figure 5. XPS results of O1s lines and Fe Fe2p3/2 lines for (a) untreated particles and (b) treated particles.
water of crystallization, as in FeCl3∙6∙H2O, since this compound is formed on the γ-Fe2O3 cores, when these are synthesized by a similar, chemically induced transition method in FeCl2 solution [15].
4. Discussion
4.1. The Structure of the Particles
The experimental results and analysis above reveal that both untreated and treated particles are spherical nanoparticles with sizes of the order of 10 nm. The untreated particles consist of γ-Fe2O3, Ni2O3 and FeCl3∙6H2O phases. There must be very little FeCl3∙6H2O since there were no clear corresponding diffraction peaks in the XRD pattern. In addition, in the treated particles, Fe(NO3)3·9H2O is present as well as the γ-Fe2O3 and Ni2O3 phases, but no FeCl3·6H2O. Comparing with the untreated particles, the proportion of Ni2O3 is lower in the treated particles. We conclude that during the treatment, FeCl3∙6H2O and some Ni2O3 are dissolved and Fe(NO3)3∙9H2O is formed instead. As a consequence, it appears that the untreated nanoparticles consist of a γ-Fe2O3 core, Ni2O3 external shell and FeCl3∙6H2O outermost layer; the treated particles have the same core and external shell, but Fe(NO3)3∙9H2O outermost layer. A schematic structural diagram of the particles is presented in Figure 6. It appears that the γ-Fe2O3 core remains unchanged when the particles are treated in Fe(NO3)3 solution.
4.2. The Molar Percentages of the Phases in the Particles
The structural data and the quantitative elemental analysis from the EDX measurements provide a description of the molar ratios of the phases for both particles, as follows.
For the untreated particles, based on γ-Fe2O3, Ni2O3 and FeCl3∙6H2O phases, the molar ratio between the γ-Fe2O3 and Ni2O3 phases,  , can be written as
, can be written as
 (1)
(1)
where aFe, aNi and aCl are the atomic percentages of Fe, Ni and Cl, respectively. Likewise, the ratio between the

Figure 6. Schematic model of the structures of the particles.
γ-Fe2O3 and FeCl3 phases,  , can be written as
, can be written as
 (2)
(2)
Consequently, the molar percentages of γ-Fe2O3 , Ni2O3
, Ni2O3 and FeCl3
and FeCl3 can be obtained as
can be obtained as
 (3)
(3)
From the EDX data listed in Table 1, the values of ,
,  and
and  can be calculated and these are given in Table 3.
can be calculated and these are given in Table 3.
For the treated particles based on γ-Fe2O3, Ni2O3 and Fe(NO3)3·9H2O phases, 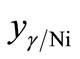 , can be written as
, can be written as
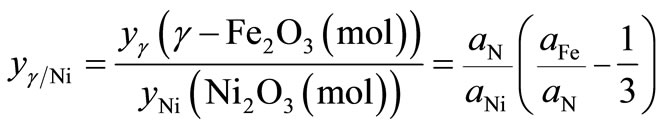 (4)
(4)
where aFe, aNi and aN are the atomic percentages of Fe, Ni and N, respectively. Likewise, the ratio between γ-Fe2O3 and Fe(NO3)3 phases, ![]() , can be written as
, can be written as
 (5)
(5)
Thus, the molar percentages of γ-Fe2O3 , Ni2O3
, Ni2O3 and Fe(NO3)3
and Fe(NO3)3 for the treated particles, can be obtained from
for the treated particles, can be obtained from
 (6)
(6)
The values of ,
,  and
and 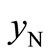 can be calculated from the data listed in Table 1, and are also listed in Table 3. Clearly, the
can be calculated from the data listed in Table 1, and are also listed in Table 3. Clearly, the  value of the treated particles (91.48/4.17) is larger than that of the untreated particles (91.74/5.63), which confirms the reduction of the Ni2O3 phase after the treatment, since the molar concentrations of γ-Fe2O3 in both untreated and treated particles can be regarded as equal.
value of the treated particles (91.48/4.17) is larger than that of the untreated particles (91.74/5.63), which confirms the reduction of the Ni2O3 phase after the treatment, since the molar concentrations of γ-Fe2O3 in both untreated and treated particles can be regarded as equal.
4.3. The Mass Percentages of the Phases in the Particles
From the molar percentage of each phase, the mass percentages of the phases in both untreated and treated particles, zi, can be deduced from
 (7)
(7)
where yi is the molar percentage and Ai is molecular weight of the i phase in the particles. Accordingly, the mass percentage of each phase for the two particles can

Table 3. The molar percentage of each phases in the untreated and treated particles yi.
be obtained from the results listed in Table 3 and the molecular weights of γ-Fe2O3, Ni2O3, FeCl3·6H2O or Fe(NO3)3·9H2O, which are listed in Table 4.
From the results in Table 4, it can be seen that the mass fractions of both γ-Fe2O3 and Ni2O3 in the treated particles are less than in the untreated particles. Therefore, the specific magnetization of the former is weaker than that of the latter, since γ-Fe2O3 is ferrimagnetic, Ni2O3 is weakly ferromagnetic [16], and both FeCl3 and Fe(NO3)3 are paramagnetic.
4.4. The Volume Percentages of the Phases in the Particles
From the mass percentage zi, the volume percentage of each phase in the particle system, ϕi, can be obtained from
 (8)
(8)
where ϕi is the density of the i phase. The densities of γ-Fe2O3, Ni2O3, FeCl3·6H2O and Fe(NO3)3·9H2O are 4.90, 5.32, 1.844 and 1.684g/cm3, respectively. Thus, from the zi values listed in Table 4, the values of ϕi can be calculated, and are listed in Table 5. For the treated particles, the volume of the γ-Fe2O3 phase should be same as for the untreated ones. The fact that the volume percentage of γ-Fe2O3 phase for the treated particles is less than that for the untreated particles shows that the size of the former is larger than that of the latter. This is in agreement with the TEM results.
4.5. The Density of the Particles
From both mass percentages and densities of each phase, the average density of the particles containing many phases, <ρ> , can be obtained from
 (9)
(9)
Using Formula (9), the average density can be estimated as 4.596 g/cm3 and 4.102 g/cm3 for the untreated and treated particles, respectively. For the treated particles, the density 4.102 g/cm3 is very close to 4.125 g/cm3, which was deduced from density measurements of the corresponding ferrofluids [12].
From the average density, the magnetization curves of M(=siρi) vs. H can be obtained from the specific magnetization, as shown in Figure 7. The values of saturation magnetization Ms(=ss· <ρ> ) were obtained from ss as 260.09 and 168.96 kA/m for the untreated and treated particles, respectively.
5. Conclusion
Using a chemically induced transition in FeCl2 solution, composite nanoparticles containing a γ-Fe2O3 core, Ni2O3 shell and FeCl3·6H2O outermost layer, were synthesized. The Fe(NO3)3 solution treatment after synthesis dissolves FeCl3 and some of the Ni2O3 to give a new Fe(NO3)3·9H2O layer on the undissolved part of the nanoparticles. This shows that during the Fe(NO3)3 treatment, it is easily absorbed on the ferrite particles to form a Fe(NO3)3·9H2O surface layer, a result similar to Fe3O4 when treated with Fe(NO3)3 [17,18]. For the composite nanoparticles with many phases, combining bulk with surface analysis using EDX, XRD and XPS reveals their structure and the molar, mass and volume percentages of each phase can be calculated. As a consequence,

Table 4. The mass percentage of each phases in the untreated and treated particles zi.

Table 5. The percentage volume of each phases in the untreated and treated particles ϕi.

Figure 7. The magnetization curves of (a) untreated particles and (b) treated particles.
the difference of both magnetization and size between the treated and untreated particles can be explained from the phase ratios. In addition, the average density of the particles can be obtained, which is very important for the synthesis of ferrofluids with accurate volume fractions of particles. The experiments indicated that after treatment with Fe(NO3)3, although the γ-Fe2O3 core is unchanged, the magnetization of the treated particles becomes much weaker than that of the untreated particles. Clearly, this is evidence showing that the surfaces of magnetic nanoparticles play an important role in determining their magnetization behavior.
6. Acknowledgements
Financial support for this work was provided by the Nature Science Foundation of China (11074205)
REFERENCES
- J. Nogués, J. Sort, V. Langlais, V. Skumryev, S. Suriñach, J. S. Muñoz, et al., “Exchange Bias in Nanostructures,” Physics Reports-Review Section of Physics Letters, Vol. 422, No. 3, 2005, pp. 65-117.
- M. Knobel, W. C. Nunes, L. M. Socolovsky, E. De Biasi, J. M. Vargas and J. C. Denardin, “Superparamagnetism and Other Magnetic Features in Granular Materials: A Review on Ideal and Real Systems,” Journal of Nanoscience and Nanotechnology, Vol. 8, No. 6, 2008, pp. 2836-2857.
- D. V. Szabó and D. Vollath, “Nanocomposites from Coated Nanoparticles,” Advanced Materials, Vol. 11, No. 15, 1999, pp. 1313-1316. http://dx.doi.org/10.1002/(SICI)1521-4095(199910)11:15<1313::AID-ADMA1313>3.0.CO;2-2
- Q. X. Liu, Z. H. Xu, J. A. Finch and R. Egerton, “A Novel Two-Step Silica-Coating Process for Engineering Magnetic Nanocomposites,” Chemistry of Materials, Vol. 10, No. 12, 1998, pp. 3936-3940. http://dx.doi.org/10.1021/cm980370a
- R. E. Rosensweig, “Ferrohydrodynamic,” Cambrige University, Combridge, 1985.
- R. Massart, “Preparation of Aqueous Magnetic Liquids in Alkaline and Acidic Media,” IEEE Transactions on Magnetics, Vol. 17, No. 2, 1981, pp. 1247-1248. http://dx.doi.org/10.1109/TMAG.1981.1061188
- F. A. Tourinho, R. Franck and R. Massart, “Aqueous Ferrofluids Based on Manganese and Cobalt Ferrites,” Journal of Materials Science, Vol. 25, No. 7, 1990, pp. 3249-3254. http://dx.doi.org/10.1007/BF00587682
- M. H. Sousa, F. A. Tourinho, J. Depeyrot, G. J. da Silva and M. C. F. Lara, “New Electric Double-Layered Magnetic Fluids Based on Copper, Nickel, and Zinc Ferrite Nanostructures,” The Journal of Physical Chemistry B, Vol. 105, No. 6, 2001, pp. 1168-1175. http://dx.doi.org/10.1021/jp0039161
- J. Crangle, “The Magnetic Properties of Solids,” Edward Arnold, London, 1977.
- B. Huke and M. Lücke, “Magnetic Properties of Colloidal Suspensions of Interacting Magnetic Particles,” Reports on Progress in Physics, Vol. 67, No. 10, 2004, pp. 1731- 1768. http://dx.doi.org/10.1088/0034-4885/67/10/R01
- Q. M. Zhang, J. Li, Y. Q. Lin, X. D. Liu and H. Miao, “The Preparation and Characterization of Ni-Fe Bioxide Composite Nanoparticles,” Journal of Alloys and Compounds, Vol. 508, No. 2, 2010, pp. 396-399. http://dx.doi.org/10.1016/j.jallcom.2010.08.065
- L. H. Lin, J. Li, J. Fu, Y. Q. Lin and X. D. Liu, “Preparation, Magnetization, and Microstructure of Ionic Ferrofluids Based on Gamma-Fe2O3/Ni2O3 Composite Nanoparticles,” Materials Chemistry and Physics, Vol. 134, No. 1, 2012, pp. 407-411. http://dx.doi.org/10.1016/j.matchemphys.2012.03.009
- R. Arulmurugan, G. Vaidyanathan, S. Sendhilnathan and B. Jeyadevan, “Co-Zn Ferrite Nanoparticles for Ferrofluid Preparation: Study on Magnetic Properties,” Physica BCondensed Matter, Vol. 363, No. 1-4, 2005, pp. 225-231. http://dx.doi.org/10.1016/j.physb.2005.03.025
- C. G. Granqvist and R. A. Buhrman, “Ultrafine Metal Particles,” Journal of Applied Physics, Vol. 47, No. 5, 1976, pp. 2200-2219. http://dx.doi.org/10.1063/1.322870
- L. L. Chen, J. Li, Y. Q. Lin, X. D. Liu, L. H. Lin and D. C. Li, “Surface Modification and Characterization of γ- Fe2O3 Nanoparticles Synthesized by Chemically-Induced Transition,” Materials Chemistry and Physics, Vol. 141, No. 2-3, 2013, pp. 828-834.
- Q. M. Zhang, J. Li, H. Miao and J. Fu, “Preparation of Gamma-Fe2O3/Ni2O3/FeCl3(FeCl2) Composite Nanoparticles by Hydrothermal Process Useful for Ferrofluids,” Smart Materials Research, Vol. 2011, 2011, Article ID: 351072. http://dx.doi.org/10.1155/2011/351072
- A. R. Wang, J. Li and Y. Wang, “Analysis of Fe3O4 Nanoparticles Treated with Fe(NO3)3,” Journal of Instrumental Analysis, Vol. 25, No. 6, 2006, pp. 35-38. (in Chinese)
- J. Li, X. Y. Qiu, Y. Q. Lin, X. D. Liu, R. L. Gao and A. R. Wang, “A Study of Modified Fe3O4 Nanoparticles for the Synthesis of Ionic Ferrofluids,” Applied Surface Science, Vol. 256, No. 23, 2010, pp. 6977-6981. http://dx.doi.org/10.1016/j.apsusc.2010.05.009
NOTES
*Corresponding author.

