American Journal of Plant Sciences
Vol.4 No.5A(2013), Article ID:32261,7 pages DOI:10.4236/ajps.2013.45A012
Efficient, Season-Independent Seed Germination in Black Cohosh (Actaea racemosa L.)
![]()
1Plant Protection, DuPont Agricultural Biotechnology, Wilmington, USA; 2Institute for Bioscience and Biotechnology Research, Fischell Department of Bioengineering, University of Maryland, Rockville, USA; 3The North Carolina Arboretum, University of North Carolina Affiliate Campus, Asheville, USA.
Email: eisenstein@umd.edu
Copyright © 2013 Bhavneet Kaur et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 12th, 2013; revised April 20th, 2013; accepted May 14th, 2013
Keywords: Actaea racemosa; Seed Germination; Morphophysiological Dormancy; Stratification; Radicle
ABSTRACT
Seed germination in black cohosh was systematically examined in eighteen populations including 15 USDA accessions with an effective protocol for the consistent, season independent germination of this valuable alternative specialty crop. Two in vitro approaches were investigated for breaking the complex double dormancy of black cohosh seeds for yearround germination of plants for increased cultivation and laboratory studies. The first approach was a two-step alternating temperature stratification in which surface sterilized seeds were incubated in darkness at 25˚C for two weeks followed by incubation at alternating temperatures of 20˚C and 8˚C for 12-hour periods with a 16-hour photoperiod for 12 months. The second was a three-step-approach that involved initial stratification of seeds in darkness at 25˚C for two weeks, followed by incubation at 4˚C in darkness for 3 - 4 months and then cultivation at 25˚C with a 16-hour photoperiod to generate seedlings. Although both approaches broke double dormancy for black cohosh seed germination, the three-step-stratification technique yielded higher percentage seed germination in less time when compared to the two-step scheme, including for seeds stored over two years. Additional factors of critical importance for efficient germination included the selection of healthy and viable seeds, as well as thorough but non-excessive surface sterilization to control bacterial and fungal contamination. The in vitro approach for black cohosh germination allowed year-round cultivation and culture of a number of different genotypic accessions to enable laboratory based studies on cell culture and transformation approaches to aid in deciphering gene-metabolite relationships in this important medicinal plant.
1. Introduction
Black cohosh (Actaea racemosa L.; syn. Cimicifuga racemosa, Nutt.) is a perennial plant of the buttercup family (Ranunculaceae) that has become a popular phytomedicinal botanical supplement used to mitigate hot flashes, mood changes, heart pounding, insomnia, bone loss and other distressing symptoms associated with perimenopause [1] and menopause [2]. The recognition of black cohosh, which apparently lacks estrogenic activity, as a natural alternative to Hormone Replacement Therapy led to a significant increase in its demand and, since 2008 it has been one of the top-ten selling herbal dietary supplement in the United States with total sales of more than $8 million [3]. Although a large number of secondary metabolites have been identified from roots and rhizomes, a mechanism for black cohosh containing extracts is unknown, and even controversial [1,4,5], with recent evidence suggesting a serotonergic mechanism of action [6].
Increases in the use of rhizomes and roots as raw material for manufacturing natural product based dietary supplements has led to overharvesting of black cohosh from the wild. Diminished populations of black cohosh due to wild-harvesting pressure has resulted in its endangered status in Illinois and Massachusetts in addition to inclusion on the United Plant Savers “At-Risk” list, and consideration for CITES Appendix II listing [7]. Long-term research undertaken by the US Forest Service, US Fish and Wildlife Service and the Garden Club of America is focused on determining sustainable harvest levels of black cohosh [8].
Cultivation of black cohosh can compensate for increased wild harvesting and can be readily accomplished by rhizome division at suitable forest sites, mass micropropagation by tissue culture, and propagation by seed. Propagation by seed has several advantages over vegetative propagation of plants. Seeds enable many plants to be produced from a single plant, the genetic variation of a population can be preserved, seedlings are typically more robust and disease-resistant than plants obtained from rhizome cuttings and resistant germplasm can be conserved [9]. In addition, seedlings are a ready source of young plant material for in vitro studies.
Seed germination in black cohosh has been challenging because the embryo exhibits deep simple epicotyl morphophysiological dormancy at maturity [10,11]. Thus, the underdeveloped embryo is physiologically dormant, a characteristic of members of Ranunculaceae. Specific temperature treatments are required in order to overcome such dormancy [12,13]. The mechanism by which low temperature expedites germination is not well understood, but a rise in gibberellin-like substances and decline in inhibitors such as abscisic acid following stratification have been implicated in the germination of seeds of peach, apple, Panax quinquefolium and yellow cedar [14- 16]. In addition a comparison of protein profiles of dormant with non-dormant chilled embryos in Acer saccharum and sugar pine seeds have revealed changes in proteins of unknown identity [17,18].
Previous studies of black cohosh have revealed sporadic and inconsistent germination in seeds sown in soil [9,19,20]. Black cohosh seeds, sown in soil during the fall, germinate in the spring after experiencing cold stratification over winter. If seeds fail to germinate and do not overcome dormancy in spring, they typically require warm-cold stratification for another season and germinate the following spring. Thus, they are also known as two-year seeds. It is thought that the dormancy of black cohosh seeds is overcome sequentially, with warm stratification initially required to break radicle dormancy, followed by cold stratification for cotyledon emergence [19].
In an effort to address the need to increase plant propagation for natural populations, as well as to enable laboratory studies using transformation and modern functional genomics to elucidate the genetic and environmental factors that control bioactive metabolite production, we sought to develop a robust approach for seed germination that decreases the lag period and accelerates the dormancy break for black cohosh. Here we report an efficient and season-independent in vitro approach for black cohosh seed germination that yields up to 80% radicle emergence in less than four months, and which has been used to achieve high rates of germination in all seed populations tested, including certified USDA accessions. The approach provides a basis for undertaking increased cultivation of black cohosh from seed on a year round basis. The approach described for generating young tissues from germinated seedlings has been instrumental in developing a transformation system and plant cell cultures for the application of modern functional genomics approaches to the study of biosynthesis of bioactive secondary metabolites in black cohosh [21].
2. Materials and Methods
2.1. Collection of Plant Material
Black cohosh seeds were collected or harvested in 2008 and 2009 from a variety of different sources. Eighteen different seed populations were used for germination studies, including: 15 USDA-certified accessions (AR-USDA) (USDA, ARS, NCRPIS, Iowa State University, Ames, IA); Crummies Creek Tree Farm, West Virginia (ARWV); harvested on-site from plants obtained from Alabama (AR-AL); and from North Carolina (AR-NC) All seeds were either refrigerated or stored at 4˚C in a (dark) environmental room.
2.2. Seeds for in Vitro Germination Experiments
Black cohosh seeds exhibit a half-moon/crescent shape (Figure 1(a)) with an average length of about 3 mm [20]. The length and width of 20 seeds from each seed lot was measured before imbibition using an AxioVison dissecting microscope (Axiovert 40CFL, Zeiss). The length was measured along the flat part of the crescent and the width was measured from the center of the flat part to the farthest point on the crescent side (Figure 1(a)). Black cohosh seeds are borne in two rows within follicles (fruits) and each follicle bears 10 - 12 seeds arranged in two rows. The seeds that appeared healthy (firm; white when longitudinally-sectioned Figure 1(b)) and sunk during imbibition were used for germination experiments. The seeds that remained on the water surface after 2 h were considered non-viable, and were found to be either empty (hollow) or senescent when dissected longitudinally (Figure 1(c)) and were discarded. Several seeds were dissected longitudinally after imbibition to visualize the embryo, and exhibited the classic heart-shaped embryo (Figure 1(d)), that is expected to give rise to germination [12, 13,20].
2.3. Surface Sterilization of Seeds and Experimental Design for in Vitro Germination Experiments
The seeds for each experiment were washed thoroughly for 15 minutes with tap water and then three times with deionized water, imbibed for two hours in sterile deionized water containing antibacterial soap (two drops per 100 mL of water) and then rinsed thoroughly with deionized water to remove all traces of soap. In a laminar flow air cabinet, the seeds were then surface sterilized for

Figure 1. Morphological characteristics of black cohosh seeds and embryos throughout germination. (a) Freshly harvested crescent-shaped black cohosh seeds; (b) Longitudinally dissected healthy seeds showing a white endosperm; (c) Non-viable hollow/brown senescent seeds; (d) Heart-shaped embryo at dehiscence; (e) Torpedo-staged embryo after warm stratification at 25˚C and one month of cold stratification; (f) Late torpedo/early cotyledonary-stage embryo after two months of cold stratification; (g) Germinating seed showing cotyledon (top arrow) and radicle (bottom arrow) three months after cold stratification; (h) Early cotyledonary-stage senescent embryo in seeds that did not germinate after three months of cold stratification; (i) Developing seedlings four months after cold stratification; (j) Two-month-old seedlings. Arrows in panels d, e, f, g and h highlight various states of embryo development. Scale: bar = 1 mm in panels b and c; bar = 100 µm panels d, e, f and h; bar = 0.5 mm in panel g.
20 minutes with 6.5% sodium hypochlorite (10% - 15% available chlorine), followed by five rinses of two minutes each with sterile deionized water. Suitable concentrations of bleach to control contamination were determined in separate experiments employing 2% - 10% solutions. Ethanol (70%) was not found to be suitable as a sterilization agent for black cohosh seeds over the course of germination experiments.
Preliminary experiments to examine the use of varying substrata such as sterile wet filter papers, MS medium [22] and water agar or pre-treatment with germination promoting compounds such as gibberellic acid or potassium nitrate were also undertaken to assess their ability to decrease the lag period and accelerate the break in dormancy of black cohosh seeds. Germination conditions were optimized in experiments performed in triplicate using 20 seeds from each lot, and scored for germination as evidenced by radicle emergence.
2.4. In Vitro Stratification
Two distinct but related approaches were investigated for breaking the double dormancy of black cohosh seeds. Experiments using various lots of seeds involved placing them in Petri dishes under conditions described and observing for bacterial or fungal contamination every day for two weeks and then twice every month for radicle emergence.
2.4.1. Alternating Temperature Stratification
An alternating temperature regime was used to assess germination efficiency. This approach involved an initial warm stratification of surface sterilized seeds at 25˚C for two weeks, followed by incubation in a growth chamber that alternated temperature between 20˚C and 8˚C. Each temperature was held for 12 h, with the warmer regime associated with a 16 h photoperiod.
2.4.2. Three-Step Stratification
An alternative, three-step approach was developed to address shortcomings of the alternating temperature stratification. The three-step stratification was adapted from Cech [9] that included a warm stratification of surfacesterilized seeds at 25˚C for two weeks, followed by cold stratification at 4˚C for three months (or until radicle and epicotyl emergence). Seeds that exhibited both radicle and cotyledon emergence were transferred to 25˚C for further growth and development of seedlings (adapted from [9]).
3. Results
3.1. Assessment of Optimal Germination Conditions
Because of the low, inconsistent yields and the significant time required for germination of black cohosh, preliminary analyses of optimal conditions were explored. Twenty seeds from each source on water agar were used in germination experiments (three replicates, n = 60). Each experiment/treatment was repeated five times (for ARAL and AR-USDA) or three times (for AR-WV and ARNC, due to contamination issues).
As can be seen in Table 1, after about three months, only a very low percentage of black cohosh seeds germinated using the alternating-temperature stratification, whereas the three-step stratification approach performed better in our laboratory. Only about 10% germination was observed across all seed lots with the alternating temperature approach, whereas between two-thirds and three-quarters of all seeds exhibited radicle emergence with the three-step approach over the same time period. After 12 months, significant germination was found using the alternating temperature regime with radicle emergence after 5 - 7 months of incubation. However, a greater extent of seeds germinated faster with the three-step stratification approach, which was consistent in all seed lots used in the study. Variation in substratum or pre-germination priming with gibberellic acid did not accelerate or affect the extent of germination significantly (data not shown). Pre-treatment with potassium nitrate induced deeper dormancy, and resulted in virtually no observable seed germination. Seeds that were surface-sterilized with 70% alcohol did not germinate at all; alcohol treatment may have killed the embryo.
3.2. Seed and Embryo Morphology during Germination
Seeds from all lots varied slightly within and among the groups in both length and width. Seed length varied from 2.01 - 3.32 mm whereas the range in width was 0.8 - 2.33 mm. Qualitative visual inspection suggested that larger seeds showed a greater extent of radicle emergence than the smaller, narrower seeds. It was very difficult to dissect the smaller and narrower seeds, even after imbibition, and hence the embryo morphology in such seeds could not be observed.
A subset of larger (3 mm length and 1.5 mm width) seeds was dissected after imbibition to reveal the classic heart shaped embryo characteristic of black cohosh (Figure 1(d)), or an early torpedo-stage embryo (Figure 1(e)). Some seeds contained a liquid mass rather than a rudimentary embryo at the time of dehiscence. It is probable that these seeds would not be able to complete the germination process, even after the temperature stratification treatment, due to retarded embryo growth and development. The morphological attributes of A. racemosa embryos changed remarkably as the development continued from heart-stage embryo to cotyledonary-stage. After two months of stratification, viable seeds exhibited a torpedo stage of the embryo (Figure 1(f)), which transitioned to the cotyledonary embryo after another month (Figure 1(g)). The embryo axis continued elongation leading to radicle emergence and later epicotyl emergence (Figure 1(i)).
Interestingly, examination of seeds that failed to germinate showed the presence of a brownish senescent embryo arrested at the late torpedo/early cotyledonary stage (Figure 1(h)), and contained significant unused endosperm. This is in contrast to normal seed development, in which the embryo continued to grow, expending the entire endosperm and culminated in radicle emergence (Figure 1(g)).
3.3. Germination of Seeds at Different Temperature Regimes
Temperature is the major factor that controls dormancy break, and it is critical for black cohosh seeds to undergo specific temperature stratification treatment for completion of germination events, with warm stratification particularly important for radicle emergence. In a group of control seeds that were not subjected to warm temperature treatment, no germination was recorded even after 18 months of incubation at 4˚C. However, incubation at 25˚C alone did not result in radicle emergence. Chilling was required after warm stratification to break both radicle and epicotyl dormancy.
The time course of the two stratification approaches on the germination of black cohosh can be seen in Figures 2(a)-(d). Radicle dormancy was broken first, followed
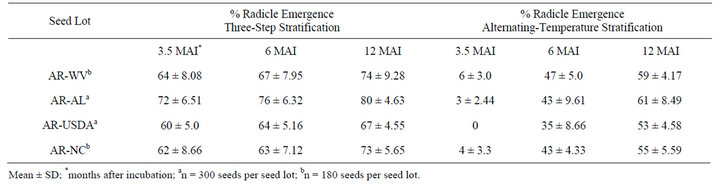
Table 1. Effect of temperature on radicle emergence in black cohosh seeds.
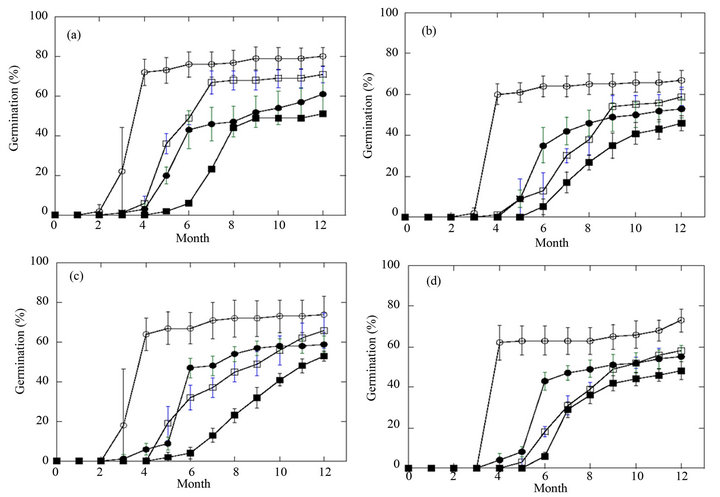
Figure 2. Germination of black cohosh seeds with an alternating temperature or three-step stratification. Percentage of germinated seeds was evaluated throughout the specific stratification process and results recorded as described under Methods. (a) AR-AL seeds, n = 300; (b) AR-USDA seeds, n = 300; (c) AR-WV seeds, n = 180; (d) AR-NC seeds, n = 180. For all panels: radicle emergence from three-step stratification (¡); radicle emergence from the alternating temperature approach (o); cotyledon emergence from three-step stratification (l); cotyledon emergence from alternating temperature regime (■).
by epicotyl emergence. Although both protocols were successfully employed to overcome the double dormancy seen for black cohosh seeds, the three-step stratification protocol resulted in radicles emerging from more seeds within a shorter time than the alternating temperature approach. The three-step stratification has been replicated with many accessions over a three-year period with high reproducibility. For example, AR-AL seeds showed 80% germination with the three-step stratification whereas the same seed lot showed only 61% germination with the alternating temperature protocol after 12 months. This difference may be attributable to seasonal variation, since germination experiments beginning in the fall coincide with the natural time for sowing seeds, whereas our experiments were conducted throughout the year, and there may be cyclical variations beyond control. Most seeds (>90%) that exhibited radicle emergence also showed cotyledon emergence. Two-month-old seedlings exhibiting true leaves are shown in Figure 1(j).
4. Discussion
In an effort to address the challenge of decreased natural plant populations, and to provide a ready supply of healthy plants in a nursery environment for cell culture and transformation, a reliable, effective and efficient germination regime for black cohosh was developed. Both a three-step-stratification and alternating temperature stratification for germination of black cohosh seeds were successful in generating plantlets. However, the threestep approach, involving an initial incubation of 25˚C for two weeks, followed by three months at 4˚C and then returned to 25˚C for seedling growth, was found to be more efficient in breaking early dormancy in black cohosh and yielding efficient, synchronous and season-independent germination. The selection of healthy seeds in combination with an effective bleach sterilization protocol is critical to attain high germination rates and minimize contamination.
The three-step stratification described here has been replicated with many accessions and highly reproducible results were observed within specific seed lots. Interestingly, some variation was observed with applying this approach from one seed lot to another (Table 1). For example, there was a difference between the extent of germination observed for AR-USDA (67%) and that seen for AR-AL (80%) after 12 months. This difference and others, may be attributable to genotypic differences or storage conditions. The slight differences in the time required for radicle emergence and cotyledon emergence may also be influenced by the genotype. The optimized approach described here was developed with freshly harvested seeds since previous investigations indicate that storage of A. racemosa seeds for more than 270 days induces a deeper dormancy in viable seeds, which may be very difficult to break [23]. Although most of the seeds used in this study were collected in 2008, high percentage radicle emergence was observed with the three-step stratification approach even after 12 months of storage at 4˚C, with more than 60% of seeds displaying a break in dormancy and radicle emergence within three to four months. On the other hand, fresh seeds exhibited the highest germination levels. Bacterial and fungal contamination among seed lots was obviously detrimental to germination, and longer storage resulted in longer times required to break dormancy. Although previous investigations have suggested a positive role for gibberellic acid treatment for embryo development [24], various pretreatment of seeds with gibberellic acid did not detectably influence germination in our hands, similarly to results observed in another study testing gibberellic acid treatment on black cohosh seed germination [20]. Optimal results were obtained with seeds used soon after dispersal, which minimized the induction of secondary dormancy and maximized germination levels. Although there were consistent differences noted between the two approaches, both in vitro methods were effective in overcoming the double dormancy in black cohosh seed germination.
The three-step stratification approach has been applied successfully for a considerable range in black cohosh seed accessions over the course of several years, yielding reasonably rapid, high percentage and season-independent germination. The approach appears independent of genotype, and relies on temperature stratification to overcome the low yields of plants due to double dormancy. This method has been used to generate a robust collection of seedlings for transplantation with a high survival rate, and for use as young plant material in studies aimed at developing cell culture, transformation and functional genomics approaches to study gene-metabolite relationships in this important non-model medicinal plant [21].
5. Acknowledgements
We thank Dr. James F. Parsons, Dr. Martin J. Spiering and Mr. Ryan Cooper for technical advice, helpful discussions and assistance during the course of these studies. This work was supported by United States Department of Agriculture Grant USDA 2008-38922-19537 and the Appalachian Center for Ethnobotanical Studies.
REFERENCES
- F. Borrelli and E. Ernst, “Black Cohosh (Cimicifuga racemosa) for Menopausal Symptoms: A Systematic Review of Its Efficacy,” Pharmacological Research, Vol. 58, No. 1, 2008, pp. 8-14. doi:10.1016/j.phrs.2008.05.008
- N. R. Farnsworth and G. B. Mahady, “Research Highlights from the UIC/NIH Center for Botanical Dietary Supplements Research for Women’s Health: Black Cohosh from the Field to the Clinic,” Pharmaceutical Biology, Vol. 47, No. 8, 2009, pp. 755-760. doi:10.1080/13880200902988637
- C. Cavaliere, P. Rea, M. E. Lynch and M. Blumenthal, “Herbal Supplement Sales Experience Slight Increase in 2008,” HerbalGram, Vol. 82, 2009, pp. 58-61.
- P. Amato and D. M. Marcus, “Review of Alternative Therapies for Treatment of Menopausal Symptoms,” Climacteric, Vol. 6, No. 4, 2003, pp. 278-284.
- R. Teschke and A. Schwarzenboeck, “Suspected Hepatotoxicity by Cimicifugae racemosae Rhizoma (Black Cohosh, Root): Critical Analysis and Structured Causality Assessment,” Phytomedicine, Vol 16, No. 1, 2009, pp. 72-84. doi:10.1016/j.phymed.2008.09.009
- S. L. Powell, T. Godecke, D. Nikolic, S. N. Chen, S. Ahn, B. Dietz, N. R. Farnsworth, R. B. van Breemen, D. C. Lankin, G. F. Pauli and J. L. Bolton, “In Vitro Serotonergic Activity of Black Cohosh and Identification of NMethylserotonin as a Potential Active Constituent,” Journal of Agricultural and Food Chemistry, Vol. 56, No. 24, 2008, pp. 11718-11726. doi:10.1021/jf803298z
- M. L. Predny, P. De Angelis and J. L. Chamberlain, “Black Cohosh (Actaea racemosa): An Annotated Bibliography,” General Technical Report SRS-97, 2006.
- J. L. Chamberlain, “Appalachian Opportunities: Medicinal and Aromatic Plants-Producing, Using and Marketing Herbs and Non-Timber Forest Products,” Proceedings of the Fourth Annual Symposium on Conserving the Appalachian Medicinal Plant Industry, Beckley, 2005, pp. 5- 16.
- R. Cech, “Growing At-Risk Medicinal Herbs: Horizon Herbs,” Williams, 2002.
- C. C. Baskin and J. M. Baskin, “Germination Ecophysiology of Herbaceous Plant Species in Tempreate Regions,” American Journal of Botany, Vol. 75, No. 2, 1988, pp. 286-305. doi:10.2307/2443896
- C. C. Baskin and J. M. Baskin, “Seeds: Ecology, Biogeography and Evolution of Dormancy and Germination,” Academic Press, San Diego, 2001.
- Y. Niimi, D.-S. Han and S. Abe, “Temperatures Affecting Embryo Development and Seed Germination of Christmas Rose (Helleborus niger) after Sowing,” Scientia Horticulturae, Vol. 107, No. 3, 2006, pp. 292-296. doi:10.1016/j.scienta.2005.08.007
- T. Nomizu, Y. Nimi and E. Watanabe, “Embryo Development and Seed Germination of Hepatica nobilis Schreber var. japonica as Affected by Temperature after Sowing,” Scientia Horticulturae, Vol. 99, No. 3-4, 2004, pp. 345-352. doi:10.1016/S0304-4238(03)00115-8
- T. J. Gianfagna and S. Rachmiel, “Changes in Gibberllins-Like Substances of Peach Seed during Stratification,” Physiologica Planarum, Vol. 66, No. 1, 1986, pp. 154- 158. doi:10.1111/j.1399-3054.1986.tb01249.x
- G. Ren, F. Chen, H. Lian, J. Zhao and X. Gao, “Changes in Hormone Content of Panax quinquefolium Seeds during Stratification,” The Journal of Horticultural Science and Biotechnology, Vol. 72, No. 6, 1997, pp. 901-906.
- N. Schmitz, S. R. Abrams and A. R. Kermode, “Changes in Abscisic Acid Content and Embryo Sensitivity to (+)- Abscisic Acid during the Termination of Dormancy of Yellow Cedar Seeds,” Journal of Experimental Botany, Vol. 51, 2000, pp. 1159-1162. doi:10.1093/jexbot/51.347.1159
- B. A. Hance and J. M. Bevington, “Changes in Protein Synthesis during Stratification and Dormancy Release in Embryos of Sugar Maple (Acer saccharum),” Physiologica Planarum, Vol. 86, No. 3, 1992, pp. 365-371. doi:10.1111/j.1399-3054.1992.tb01332.x
- T. L. Noland and J. B. Murphy, “Protein Synthesis and Aminopeptidase Activity in Dormant Sugar Pine Seeds during Stratification and Warm Incubation,” Journal of Plant Physiology, Vol. 124, No. 1-2, 1986, pp. 1-10. doi:10.1016/S0176-1617(86)80172-9
- J. M. Baskin and C. C. Baskin, “Epicotyl Dormancy in Seeds of Cimicifuga racemosa and Hepatica acutiloba,” Bulletin of the Torrey Botanical Club, Vol. 112, No. 3, 1985, pp. 253-257. doi:10.2307/2996540
- J.-A. H. McCoy, “Seed and Rhizome Propagation of Actaea racemosa L. (Black Cohosh) and Analysis of Associated Triterpene Glycosides,” Doctoral Dissertation, Clemson University, 2004.
- M. J. Spiering, L. A. Urban, D. L. Nuss, V. Gopalan, A. Stoltzfus and E. Eisenstein, “Gene Identification in Black Cohosh (Actaea racemosa L.): Expressed Sequence Tag Profiling and Genetic Screening Yields Candidate Genes for Production of Bioactive Secondary Metabolites,” Plant Cell Reports, Vol. 30, No. 4, 2011, pp. 613-629. doi:10.1007/s00299-010-0979-5
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497.
- M. A. Albrecht and B. C. McCarthy, “Effects of Storage on Seed Dormancy and Survivorship in Black Cohosh (Actaea racemosa L.) and Goldenseal (Hydrastis canadensis L.),” Seed Science and Technology, Vol. 35, No. 2, 2007, pp. 414-422. doi:10.1111/j.1399-3054.1962.tb08052.x
- M. Popp, R. Schenk and G. Abel, “Cultivation of Cimicifuga racemosa (L.) Nuttal and Quality of CR Extract BNO 1055,” Maturitas, Vol. 44, 2003, pp. S1-S7. doi:10.1016/S0378-5122(02)00343-2

