 Vol.3, No.9, 783-794 (2011) Natural Science http://dx.doi.org/10.4236/ns.2011.39103 Copyright © 2011 SciRes. OPEN ACCESS Kinetic parameters and thermal decomposition for Novel 1,1-malonyl-bis(4-p-Chlorophenylthiosemicarbazide) and Cu(II), Co(II), Zn(II) and Sn(II) complexes (H4pClMaTS) synthesized by electrochemical method Ragab R. Amin1*, Yamany B. Yamany2, Mohamed Abo-Aly3, Ali M. Hassan4 1Basic and Applied Science Department, Faculty of Dentistry, Nahda University, New Beni-Sueff, Egypt; *Corresponding Author: rramin2010@yahoo.com 2Faculty of Medicine and Medical Science, Taif University, Taif, Saudia Arabia; 3Chemistry Department, Faculty of Science, Ain Shams University, Cairo, Egypt; 4Chemistry Department, Faculty of Science, Al-Azhar University, Cairo, Egypt. Received 20 June 2011; revised 23 July 2011; accepted 7 August 2011. ABSTRACT Anodic oxidation of Co, Cu, Zn, and Sn metals in an acetone solution of 1,1-malonayl-bis(4-p- Chloro-phenylthiosemicarbazide) yields com- plexes of composition with general formula [Co2(pClMaTS)(H2O)6]·2H2O, [Cu2(pClMaTS)(H2O)6], [Zn2(pClMaTS)(H2O)6] and [Sn2(pClMaTS)(H2O)6]·2H2O. Chelation was inve- stigated based on elemental analysis, conducti- vity, magnetic moment, spectral (UV-Vis, IR, Raman, 1HNMR, mass), thermal, and ESR stu- dies. The Raman and infrared spectral studies suggests the tridentate behavior of the ligand from each tail. Since the ligand has two thiose- micarbazide groups, it may acts in an SNO tri- dentate fashion from each side with one of the two metal ions forming a polynuclear complex coordinating through both of the lone pair of electrons the enolic oxygen of the carbonyl group (C=O), the azomethine nitrogen (C=N) and the thioenol form of the thiocarbonyl group (C=S). The differential thermogravimetric analy- sis (DTG) curves were used to study the de- composition steps of the isolated complexes using Horowitz-Metzger (HM) and Coats-Redfern (CR) methods. The kinetic thermodynamic para- meters such as: E*, ∆H*, ∆S*and ∆G* are calcu- lated from the DTG curves. Keywords: Metal(II) Complexes; Electrochemical; Thermogravimetric; Bis-Thiosemicarbazide 1. INTRODUCTION Thiosemicarbazide and their derivatives are of current interest with respect to their uses as analytical reagents for high performance liquid chromatographic separations of metal(II) ions as metal chelates [1-3], potentiometric sensors [4], analytical detrmenation of metal ions [5] and clinical analysis [6]. Most of these compounds have an- tifungal [7], antimicrobial [8], antitumor [9], biological activity [10,11] as well as hypoglycemic effects [12-14]. In addition, thiosemicarbazide derivatives have ability to form chelate complexes with transition metal ions [15- 20]. Continuing our studies for the electrochemical syn- thesis of new metal complexes of ligands containing N, S and O atoms through the reaction of metal ions scari- fied from the anodic dissolution of metals [16,21,22]. In this paper we are reported a novel complexes isolated from the reaction between 1,1-Malonyl-bis(-4-p-chloro- phenyl thiosemicarbazide) and the metal ions scarified from the dissolution of Cobalt, Copper, Zinc and Tin metals. The modern spectroscopic investigations are used to elucidate the structure of the prepared materials. The thermal decomposition is also used to infer the stru- cture of the metal complexes and to calculate the diffe- rent thermodynamic activation parameters [23,24]. 2. EXPERIMENTAL 2.1. The Materials All the chemicals (Aldrich) were subjected to purify- cation before use. The solvents, DMF (BDH) (Analar), absolute acetone, ethanol and methanol (Fluka) used were reagent grade. The metals (Alfa Inorganics) used, Co, Cu, Zn, and Sn were purchased in the form of sheets (~2 cm × 2 cm, 2 - 3 mm thick). The oxide surface was removed by treating the metal with conc. HNO3 for se- veral minutes and then washing with distilled water. Tet-  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 784 raethylammonium perchlorate, Et4NClO4, (BDH) was used as supplied [25]. 2.2. The Organic Compounds 2.2.1. Preparation of 1,1-Malonyl-Hydrazide 1,1-Malonyl-dihydrazine was prepared by adding Ma- lonyl Chloride (7 gm 0.05 mol) to alcoholic solution of hydrazine hydrate (5 gm 0.1 mole). The reaction mix- ture was exothermic and left to cool with stirring. A brown crystal precipitate was formed and washed with ethanol diethyl ether and left to dry. Yield (5 gm 76%) and the melt point at 130˚C. 2.2.2. Preparation of 1,1-Malonyl bis-4-(p-Chlorophenylthiosemi Carbazide) 1,1-Malonyl bis-4-(p-chlorophenylthiosemicarbazide) was prepared by adding 4-chloro-phenylisothiocynate (3.4 gm, 0.02 mol) to an alcoholic solution of malonic acid dihydrazide (1.32 gm 0.01 mole). The reaction mixture was refluxed for 1 hour and left to cool with stirring. The resulting white crystals were collected and washed with ethanol and diethyl ether, respectively. The resulting solids were filtered hot, washed with hot dist. water, EtOH and dried by Et2O and finally dried in vacuo over silica gel. Yield (4.3 gm 91%) and the melt point at 200˚C [26] (Figure 1). 2.3. Electrochemical Procedure The apparatus used in the electrochemical reaction consists of a tall-form 100 mL Pyrex beaker containing 50 mL of the appropriate amount of the organic ligand dissolved in acetone solution. The cathode is a platinum wire of approximately 1 mm diameter. In most cases, the metal was suspended and supported on a platinum wire. [21,22] Measurements of the electrochemical efficiency, Ef, defined as moles of metal dissolved per Faraday of electricity, for the M/L system (where L = ligand used) gave Ef = 0.5 0.05 mol·F−1.[25]. Electrochemical Synthesis of 1,1-Malonayl bis-4-(p-Chlorophenyl Thiosemicarbazide) Metals Complexes The ligand H4pClMaTS (0.236 gm, 0.5 mmol) was dissolved in the minimum amount of DMSO (0.5 mL) followed by the addition of 50 mL of acetone and 2.5 mg of Et4NClO4. When the current 40 mA was passed through the cell for 1hour, the amount of Cobalt con- sumed is 59 mg and a dark brown precipitate was formed (the product is 0.364 gm, % yield 99.7 and Ef = 0.51). It was collected, washed with diethyl ether and dried. The resulting dark brown powder was collected and analyzed as [Co2(pClMaTS)(H2O)6]·2(H2O). By the same way Cu, Zn, and Sn complexes were isolated. H N N H S N H C H N N H S N H C Figure 1. The structure of 1,1-malonayl-bis(4-p-chlorophenyl- thio semicarbazide). 2.4. Analytical Measurements 2.4.1. Spectral Measurements The absorbances of solutions were measured in UV/ Vis range at nano-Photoenergy Central Laboratory, Ain Shams University. Infrared spectra for the samples were recorded by Perkin Elmer FTIR 1605 using KBr pellets at National Research Center, Cairo, Egypt. 2.4.2. 1H-NMR Spectra The 1H NMR spectra were recorded on an Varian Mercury VX-300 NMR spectrometer. All the spectra were run at 300 MHz was run at 75.46 MHz in deuterated di- methylsulphoxide (DMSO-d6). Chemical shifts were quoted in δ and were related to that of the solvents. 2.4.3. Microanalytical Techniques Carbon, hydrogen and nitrogen contents were deter- mined using a Perkin-Elmer CHN 2400. The copper, cobalt, zinc, and tin contents were determined gravimet- rically by the direct ignition of the complexes at 1000˚C for 3 hours till constant weight. The residue was then weighted in the forms of metal oxides. The melting point of ligand and their metal complexes were measured by the electro thermal melting point Stuart SMP3 made in UK. 2.4.4. Magnetic Measurements Magnetic measurements were carried out on a Sher- wood Scientific magnetic balance using Gouy method. Calibratio n: Hg[Co(CNS)4] and [Ni(en)3](S2O3) are easily prepared pure, do not decompose or absorb moisture and pack well. Their susceptibilities at 293 K are 16.44 × 10–6 and 11.03 × 10–6 c.g.s. Units, decreasing by 0.05 × 10–6 and 0.04 × 10–6 per degree temperature raise respec- tively, near room temperature. The cobalt compound, be- sides having the higher susceptibility, also packs rather densely and is suitable for calibrating low fields, while the nickel compound with lower susceptibility and den- sity is suitable for higher field. Here we were used Hg[Co(CNS)4] only as calibrant, Micro analytical Center, Cairo University, Egypt. 2.5. Thermal Investigation Thermogravimetric analysis (TGA and DTG) were  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 785 carried out in dynamic nitrogen atmosphere (30 ml/min) with a heating rate of 10˚C/min using a Schimadzu TGA-50H thermal analyzer. 3. RESULTS AND DISCUSSION Measurements of the electrochemical efficiency, Ef, defined as moles of metal dissolved per Faraday of elec- tricity, for the Co/L system (where L = ligand) gave Ef = 0.5 0.05 mol·F–1. The values show that the reaction of the ligand with cobalt anode is compatible with the fol- lowing steps 1 and 2. 1) The First step: 2 422 Cathode:H L2eH LHg (1) 2 22 Anode:H LCoCoH L2e (2) 2) The Second step: 2 22 Cathode:CoH L2eCoLHg (3) 2 2 Anode:CoLCoCo L2e (4) Anodic oxidation of Co, Cu, Zn, and Sn metals in an anhydrous acetone solution of 1,1-malonayl-bis (4-p- Chlorophenylthiosemicarbazide) yields complexes of composition with general formula [Co2(pClMaTS)(H2O)6]2 H2O, [Cu2(pClMaTS)(H2O)6], [Zn2(pClMaTS)(H2O)6] and [Sn2(pClMaTS)(H2O)6]·2H 2O. Structural investigation of the ligand and their complexes has been made based on elemental analysis, conductivity, magnetic moment, spe- ctral (UV-Vis, IR, Raman, 1H-NMR, Mass), thermal stu- dies. The elemental analysis and some physical data of the resulted compounds are given in Table 1. The com- plexes are air-stable, hygroscopic. All 1,1-malonayl-bis (4-p-chlorophenyl) thiosemicarbazide, Co(II), Cu(II), Zn(II) and Sn(II) complexes are paramagnetic in nature. They are white, dark brown, dark brown, yellowish white and page respectively; quite stable in atmospheric conditions; insoluble in water, ethanol and Diethylether but are completely soluble in DMF and DMSO the complexes have different melting points (200˚C - 243˚C). 3.1. Molar Conductivity The molar conductivity values of the 1,1-malonayl-bis (4-p-chlorophenylthiosemicarbazide) and its complexes in DMSO solvent (1.0 × 10–3 mol·L–1) were 20 μs for (H4pClMaTS) ligand, 37 μs for [Co2(pClMaTS)(H2O)6] 2(H2O), 35 μs for [Cu2(pClMaTS)(H2O) 6], 38 μs for [Zn2(pClMaTS)(H2O)6] and 36 μs for [Sn2(pClMaTS)(H2O)6]·2(H2O) respectively. The molar conductivity measurements located in the range of non- electrolytic behavior (Table 1). 3.2. Infrared Spectra Important IR spectral data of the ligands and com- plexes are summarized in Table 2. The IR spectrum of [H4pClMaTS] shows bands at 3310, 3196 and 3111 cm-1 for the three-NH groups present in the ligand. The bands occurring at 1657, 1400, 1341, 902 and 814 cm−1 are assigned to ν(C=O), thioamide I [β(NH) + ν(CN)], thioamide II [ν(CN) + β(NH)], ν(N-N) and ν(C=S), re- spectively. An exhaustive comparison of the IR spectra of the ligand and complexes gave information about the mode of bonding of the ligand in metal complexes. The IR spectrum of complexes [Co2(pClMaTS)(H2O)6]·2(H2O), [Cu2(pClMaTS)(H2O)6], [Zn2(pClMaTS) (H2O) 6] and [Sn2(pClMaTS)(H2O)6]·2(H2O) when compared with ligand [H4pClMaTS], indicates that bands due to ν(NH), ν(C=O) and ν(C=S) are absent, but new bands appear at 1602 and 763 cm–1 due to ν(N=C) and ν(C-S), respec- tively. Suggesting removal of both the hydrazinic pro- tons via enolisation and thioenolisation and bonding of the resulting enolic oxygen and thiolato sulfur takes place with Co (II), Cu(II), Zn(II) and Sn(II). Further- more, the ligand bands due to thioamide I, thioamide II and ν(N-N) undergo a positive shift of (16 - 40 cm–1), (59 cm–1) and (23 - 43 cm–1) respectively. The magnitude of the positive shift supports that enolic oxygen, thiolato sulfur and both hydrazinic nitrogens are involved in co- ordination and [H4pClMaTS] behaves as tetranegatively charged hexadentate species in complexes [Co2(pClMaTS)(H2O)6]2 H2O, [Cu2(pClMaTS)(H2O)6], [Zn2(pClMaTS)(H2O)6] and [Sn2(pClMaTS)(H2O)6].2H2O. IR spectral bands of com- plexes are appear of bands in the range (743 - 777 cm–1) assigned to groups (C-S) vibrations. This is also con- firmed by the appearance of bands in the range of 418 - 428 cm–1, this has been assigned to the ν(M–N) [27,28]. And the appearance of bands in the range of 490 - 501 cm–1, this has been assigned to the ν(M-O). It is due to the increase in the band strength, which again confirms the coordination via the azomethine nitrogen. The band appearing at ca. 814 cm–1 ν(C=S) and at 1657 cm–1 ν(C=O) in the IR spectral of ligand is shifted towards lower wave number. It indicates that thione sulphur and also the enolic oxygen coordinates to the metal ion [27]. Thus the ligand behaves as tridentate in bath saide che- lating agent coordinating through azomethine nitrogen, thiolate sulphur and enolic oxygen. IR spectral of ligand and its metal complexes are shown in the Figure 2. 3.3. Raman Spectra Important Raman spectral data of the ligands and its metal complexes are summarized in Table 3. The Raman spectrum of [H4pClMaTS] shows bands at 3201 cm–1 for  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 786 Table 1. Analytical Results for the Prepared Complexes of 1,1-malonayl-bis(4-p-chlorophenyl thiosemicarbazide) and their metal complexes. % Found (Calc.) Compound Empirical formula Formula weight Colour m.p. (˚C) C H N Am μs (H4pClMaTS), I C17H16Cl2N6O2S2 471.38 White 200 43.18 (43.32)3.08 (3.42) 17.6 (17.83)20 [Co2(pClMaTS)(H2O)6]·2(H2O), Ia C17H28Cl2Co2N6O10S2 729.3 Dark Brown 220 27.7 (28) 4.25 (3.87) 11.35 (11.52)37 [Cu2(pClMaTS)( H2O)6], Ib C17H24Cl2Cu2N6O8S2 702.54 Dark brown 210 29.86 (29.06)3.39 (3.44) 11.5 (11.96)35 [Zn2(pClMaTS)(H2O)6], Ic C17H24Cl2Zn2 N6O8S2 706.3 Yellowish White213 29.07 (28.91)3.30 (3.43) 11.40 (11.9)38 [Sn2(pClMaTS) (H2O)6]·2(H2O), Id C17H28Cl2Sn2 N6O10S2 848.9 Page 243 24.01 (24.05)3.22 (3.32) 9.34 (9.9) 36 Table 2. Significant IR spectral bands (cm−1) of the ligand of 1,1-malonay l-bis(4-p-chlorophenyl thiosemicarbazide) and its metal complexes. The compound Assignments I Ia Ib Ic Id υ(OH) - 3407 3435 3429 3429 υ(N4H) 3310 3237 3238 3305 3305 υ(N2H) 3196 3179 3180 3194 3197 υ(NH) 3111 3109 3111 3105 3111 CH-arom. 3005 3034 3053 3005 3007 CH-aliph. 2940 2980 2934 2938 2940 υ(C=O)/υ(NCO) 1657 1595 1599 1591 1599 Thioamide I [β(NH)+υ(CN)] 1400 1420 1431 1416 1440 Thioamide II [υ(CN)+β(NH)] 1341 1400 1400 1400 1400 δ(OH) - 1306 1310 1310 1308 υ(C-O) - 1290 1219 1273 1246 υ(N-N) 902 935 945 924 925 υ(C=S)/υ(C-S) 814 743 772 777 777 υ(M-O) - 493 501 490 490 υ(M-N) - 421 428 418 421 Table 3. Significant Raman spectral bands (cm−1) of the ligand of 1,1-malonayl-bis(4-p-chlorophenyl thiosemicarbazide) and it’s metal complexes. The compound Assignments I Ic υ(NH) 3201 3209 CH-arom. 3063 3062 CH-aliph. 2934 2933 υ(C=O)/υ(NCO) 1635 1593 Thioamide I [β(NH)+υ(CN)] 1405 1444 Thioamide II [υ(CN)+β(NH)] 1355 1395 δ(OH) - 1315 υ(N-N) 1088 1090 υ(C=S)/υ(C-S) 824 779  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 787 Figure 2. IR spectral of 1,1-Malonayl-bis(4-p-chloro phenyl- thiosemicarbazide) and its metal complexes. the NH groups present in the ligand. The bands occur- ring at 1635, 1405, 1355, 1088 and 824 cm–1 are as- signed to ν(C=O), thioamide I [β(NH) + ν(CN)], thioa- mide II [ν(CN) + β(NH)], ν(N-N) and ν(C=S), respec- tively [29]. An exhaustive comparison of the Raman spectra of the ligand and complexes gave information about the mode of bonding of the ligand in metal com- plexes. The Raman spectrum of complexes [Zn2(pClMaTS)(H2O)6]) when compared with [H4pClMaTS], indicates that bands due to ν(NH), ν(C=O) and ν(C=S) are absent, but new bands appear at ca. 1593 and 779 cm–1 due to ν(N=C) and ν(C-S), respectively. suggesting removal of both the hydrazinic protons via enolisation and thioenolisation and bonding of the result- ing enolic oxygen and thiolato sulfur takes place with Zn(II). Furthermore, the ligand bands due to thioamide I, thioamide II and ν(N-N) undergo a positive shift of (39 cm–1), (40 cm–1) and (2 cm–1) respectively. The magni- tude of the positive shift supports that enolic oxygen, thiolato sulfur and both hydrazinic nitrogens are in- volved in coordination and [H4pClMaTS] behaves as tetranegatively charged hexadentate species in com- plexes [Zn2(pClMaTS)(H2O)6]. Raman spectral bands of complexes are appear of bands at (779 cm–1) assigned to groups (C-S) vibrations. It indicates that thione sulphur and also the enolic oxygen coordinates to the metal ion [30]. Thus, it may be concluded that the ligand behaves as hexadentate chelating agent coordinating through azo- methine nitrogen and thiolate sulphur. 3.4. Electronic Spectra The electronic spectra of both 1,1-malonayl-bis(4-p- chlorophenyl thiosemicarbazide) ligand and its com- plexes were performed in DMSO and the spectral data are listed in Table 4. There are two main absorption bands in the spectra of the free ligand and their com- plexes; the first band is exhibited at the range 284 - 308 nm and assigned to π-π* [31], and the second appeared at the range 330 - 358 nm due to n-π* intraligand transi- tions [32]. These absorptions also present in the spectra of the Co(II), Cu(II) , Zn(II) and Sn(II) and [H4pClMaTS] ligand complexes, but they are shifted. In the spectra of all complexes attributed to the complexation behavior of the ligand towards metal ions which was supported the coordination of the ligand-to-metallic ions, shown as the Figure 3. 3.5. Magnetic Susceptibility Co(II) has the electronic configuration 3d* and should exhibit a magnetic moment higher than that expected for two unpaired electrons in octahedral (1 - 1.13 BM). The magnetic moment observed for the Co(II) complexes lies in the value of 1.3 BM which is consistent with the octa- hedral stereochemistry of the complexes. Room-tempe- rature magnetic moment of the Cu(II) complexes lies in the range of 1 BM, corresponding to one unpaired elec- tron. whatsoever the geometry of Cu(II) is, its complexes always show magnetic moment corresponding to one unpaired electron. 3.6. 1H-NMR Spectra Thus, the 1H-NMR spectra of the [Zn2(pClMaTS)(H2O)6] complex on comparing with those of spectrum of the free 1,1-malonayl-bis(4-p- chlorophenylthiosemicarbazide) ligand (L) indicate that. L ligand act as hexadentate ligand through the nitrogen atom of C=N azomethine group, oxygen atom of C=O carbonyl group and sulfur atom of C=S group. 1H-NMR spectra of zinc(II) complex was carried out in DMSO-d6 as a solvent, the data obtained are in agreement with the suggested coordination through the C=N and C=S groups by presence of the signals of NH, the complexes formed due to loss four protons (two from 2NH amine groups and two protons from 2NH amide groups). (H4pClO × TS) 1H-NMR δ (ppm): 9.75(6,18NH amide group), 1.95(7,19NH amine group), 3.35(CH2) 6.6 - 7.75 (CH-aromatic) [Zn2(pClO × TS)(ac)2] 2(H2O) 1H-NMR δ (ppm): 1.15 (H2O), (NH amide groups disappeared), (NH amine groups disappeared), 3.35(CH2), 5.2(10,22CNH aromatic), 6.6 - 7.75(CH-aromatic shifted).  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 788 Table 4. The electronic spectral data of1,1-malonayl-bis(4-p-chlorophen ylthiosemicarbazide) and its metal complexes. λmax nm (cm−1) Compound π-π* C=S n-π* C=S CT-transition H4pClMaTS 287 (34840) 344 (29070) [Co2(pClMaTS)(H2O)6]·2(H2O) 288 (34720) 342 (29240) 462 (21650) 485 (20620) 522 (19160) [Cu2(pClMaTS)(H2O)6] 308 (32470) 358 (27930) 466 (21460) 493 (20280) 524 (19080) [Zn2(pClMaTS)(H2O)6] 284 (35210) 344 (29070) [Sn2 (pClMaTS) (H2O)6]·2(H2O) 288 (34720) 330 (30300) Figure 3. The electronic spectral of 1,1-malonayl-bis(4-p-chloro phenylthio- semicarbazide) and its metal complexes. 3.7. Mass Spectrum The electronic impact mass spectrum of the complex [Cu2(pClMaTS)(H2O)6]{1,-malonayl-bis(4-p-chlorophen ylthiosemicarbazide) copper trihydrate} is fragment to half molecule species at m/z = 304 amu corresponding to species {4-methylene-p-chlorophenylthiosemicarbazide copper} [C9H7ClN3OSCu], which confirms the proposed formula. It also shows series of peaks at 36, 50, 75, 90, 111, 138, 152, 184, 229, 263, and 300 amu correspond- ing to various fragments. The electronic impact mass spectrum of the [Sn2(pClMaTS)(H2O)6]·2(H2O) complex shows fragment molecular ion (M+) peak at m/z = 421 amu corresponding to species [C10H9ClN3O4SSn], which confirms the proposed formula. It also shows series of peaks at 75, 111, 127, 169, 218, 302 and 336 amu corre- sponding to various fragments. The intensities of these peaks give the idea of the stabilities of the fragments. 3.8. Thermogravimetric Analysis Thermogravimetric analysis curves (TGA and DTG) of the 1,1-malonayl-bis-(4-p-chlorophenyl thiosemicar- bazide) ligand and its complexes are shown in Figure 4 and all the data are summarized in Table 5. 1,1- Malonayl-bis-(4-p-chlorophenylthiosemicarbazide) ligand was thermally decomposed in mainly decomposition steps within the temperature range successive decompo- sition steps within the temperature range 25˚C - 700˚C. The first decomposition step (obs. = 39.14%, calc. = 39%) within the temperature range 25˚C - 245˚C, may be attributed to the liberation of the 2(HNCO), 2H2S and 2(NH) fragments. The second decomposition steps found within the temperature range 245˚C - 345˚C (obs. = 32.8%, calc. = 32.5%), which is reasonably accounted by the removal of 2(HCN), (C2H2) and Cl2. The decom- position of the ligand molecule ended with a final (C17H2) residue (obs. = 28%, calc = 28.3%). The complex [Co2(pClMaTS)(H2O)6]2(H2O) was ther- mally decomposed in five successive decomposition steps within the temperature range 25˚C - 1000˚C. The first decomposition step (obs. = 5.2%, calc. = 4.94%) within the temperature range 25˚C - 188˚C, may be attri-  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 789 Figure 4. TGA and DrTGA diagram of 1,1-malonayl-bis(4-p-chlorophenylthiosemicarbazide) and its metal complexes. buted to the liberation of the two water molecules. The second decomposition steps found within the tempera- ture range 188˚C - 448˚C (obs. = 33.1%, calc. = 33.5%), which is reasonably accounted by the removal of 6water molecules 2N2, 2(HCN), and C2H2 fragments. The de- composition third step found within the temperature 448˚C - 760˚C (obs. = 23.4%, calc = 22.9%) which is reasonably accounted for by the removal of S2, Cl2 and O2 molecules. The decomposition fourth step found within the temperature 760˚C - 885˚C (obs. = 21.8%, calc = 22.5%) which is reasonably accounted for by the removal of CH4 and C12H4 fragments. The rest of the ligand molecule was removed and fifth the decomposi- tion of the Co(II) complex molecule ended with a final Co2 molecule is cobalt residue (obs. = 17.3%, calc = 16.5%). The TG curve of [Cu2(pClMaTS)(H2O)6] complex in- dicates that the mass change begins at 25˚C and con- tinuous up to 1000˚C. The first and second mass loss corresponds to the liberation of the 6water molecules (obs. = 16.4%, calc = 15.4%) within the temperature range 25˚C - 245˚C. The third decomposition step occurs in the range 245˚C - 475˚C and corresponds to the loss of N2, 2(HCN), N2H2, and O2 (obs. = 20.6%, calc = 20.5%). The decomposition fourth step found within the tem- perature 475˚C - 765˚C (obs. = 42.4%, calc = 42.5%) which is reasonably accounted for by the removal of (C13H8, S2 and Cl2) fragments. The fifth decomposition 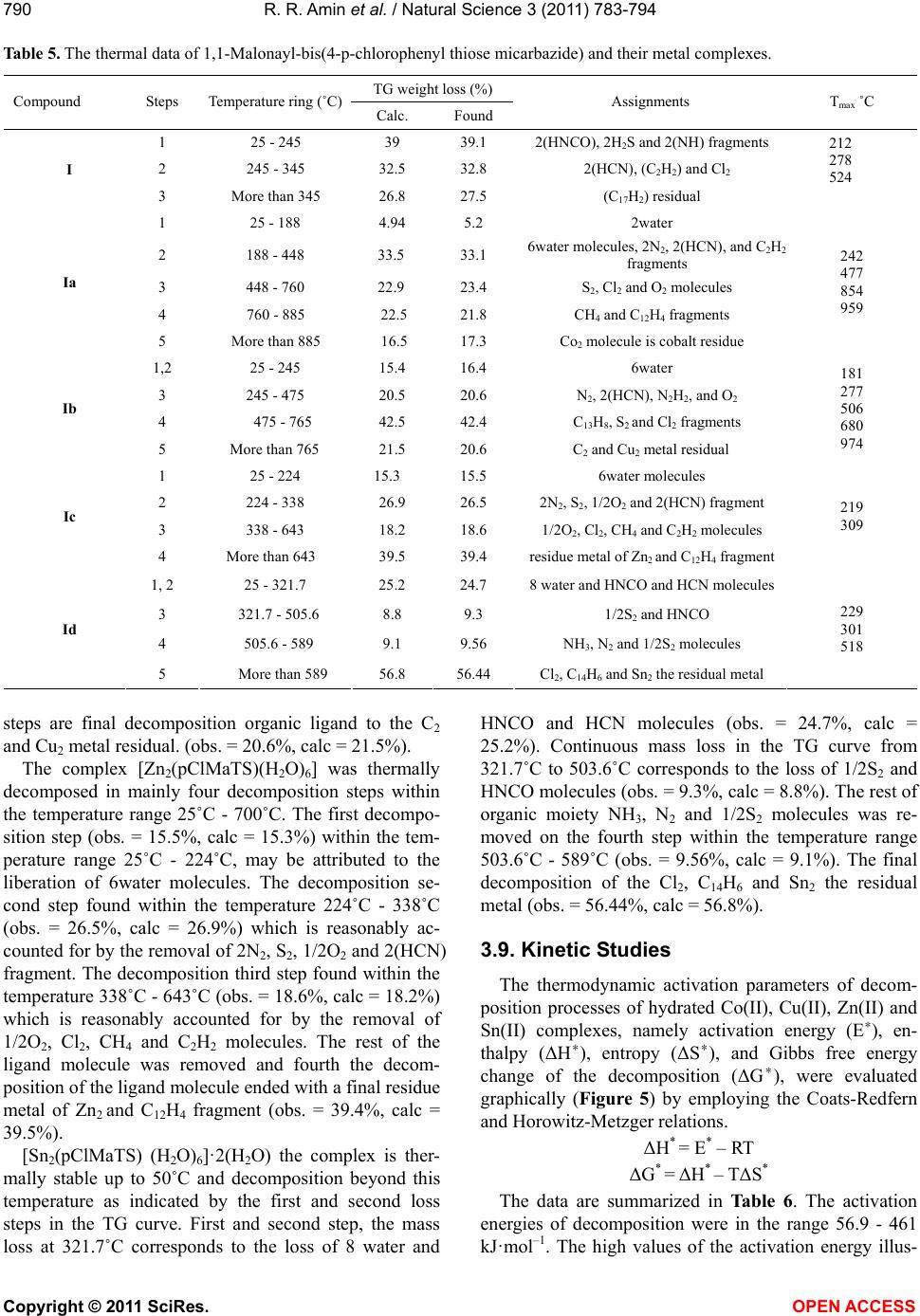 R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 790 Table 5. The thermal data of 1,1-Malonayl-bis(4-p-chlorophenyl thiose micarbazide) and their metal complexes. TG weight loss (%) Compound Steps Temperature ring (˚C) Calc. Found Assignments Tmax ˚C 1 25 - 245 39 39.1 2(HNCO), 2H2S and 2(NH) fragments 2 245 - 345 32.5 32.8 2(HCN), (C2H2) and Cl2 I 3 More than 345 26.8 27.5 (C17H2) residual 212 278 524 1 25 - 188 4.94 5.2 2water 2 188 - 448 33.5 33.1 6water molecules, 2N2, 2(HCN), and C2H fragments 3 448 - 760 22.9 23.4 S2, Cl2 and O2 molecules 4 760 - 885 22.5 21.8 CH4 and C12H4 fragments Ia 5 More than 885 16.5 17.3 Co2 molecule is cobalt residue 242 477 854 959 1,2 25 - 245 15.4 16.4 6water 3 245 - 475 20.5 20.6 N2, 2(HCN), N2H2, and O2 4 475 - 765 42.5 42.4 C13H8, S2 and Cl2 fragments Ib 5 More than 765 21.5 20.6 C2 and Cu2 metal residual 181 277 506 680 974 1 25 - 224 15.3 15.5 6water molecules 2 224 - 338 26.9 26.5 2N2, S2, 1/2O2 and 2(HCN) fragment 3 338 - 643 18.2 18.6 1/2O2, Cl2, CH4 and C2H2 molecules Ic 4 More than 643 39.5 39.4 residue metal of Zn2 and C12H4 fragment 219 309 1, 2 25 - 321.7 25.2 24.7 8 water and HNCO and HCN molecules 3 321.7 - 505.6 8.8 9.3 1/2S2 and HNCO 4 505.6 - 589 9.1 9.56 NH3, N2 and 1/2S2 molecules Id 5 More than 589 56.8 56.44 Cl2, C14H6 and Sn2 the residual metal 229 301 518 steps are final decomposition organic ligand to the C2 and Cu2 metal residual. (obs. = 20.6%, calc = 21.5%). The complex [Zn2(pClMaTS)(H2O)6] was thermally decomposed in mainly four decomposition steps within the temperature range 25˚C - 700˚C. The first decompo- sition step (obs. = 15.5%, calc = 15.3%) within the tem- perature range 25˚C - 224˚C, may be attributed to the liberation of 6water molecules. The decomposition se- cond step found within the temperature 224˚C - 338˚C (obs. = 26.5%, calc = 26.9%) which is reasonably ac- counted for by the removal of 2N2, S2, 1/2O2 and 2(HCN) fragment. The decomposition third step found within the temperature 338˚C - 643˚C (obs. = 18.6%, calc = 18.2%) which is reasonably accounted for by the removal of 1/2O2, Cl2, CH4 and C2H2 molecules. The rest of the ligand molecule was removed and fourth the decom- position of the ligand molecule ended with a final residue metal of Zn2 and C12H4 fragment (obs. = 39.4%, calc = 39.5%). [Sn2(pClMaTS) (H2O)6]·2(H 2O) the complex is ther- mally stable up to 50˚C and decomposition beyond this temperature as indicated by the first and second loss steps in the TG curve. First and second step, the mass loss at 321.7˚C corresponds to the loss of 8 water and HNCO and HCN molecules (obs. = 24.7%, calc = 25.2%). Continuous mass loss in the TG curve from 321.7˚C to 503.6˚C corresponds to the loss of 1/2S2 and HNCO molecules (obs. = 9.3%, calc = 8.8%). The rest of organic moiety NH3, N2 and 1/2S2 molecules was re- moved on the fourth step within the temperature range 503.6˚C - 589˚C (obs. = 9.56%, calc = 9.1%). The final decomposition of the Cl2, C14H6 and Sn2 the residual metal (obs. = 56.44%, calc = 56.8%). 3.9. Kinetic Studies The thermodynamic activation parameters of decom- position processes of hydrated Co(II), Cu(II), Zn(II) and Sn(II) complexes, namely activation energy (E∗), en- thalpy (ΔH∗), entropy (ΔS∗), and Gibbs free energy change of the decomposition (ΔG∗), were evaluated graphically (Figure 5) by employing the Coats-Redfern and Horowitz-Metzger relations. ΔH* = E* – RT ΔG* = ΔH* – TΔS* The data are summarized in Table 6. The activation energies of decomposition were in the range 56.9 - 461 kJ·mol–1. The high values of the activation energy illus-  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 791 Figure 5. Kinetic data curves of: (a) 1,1-Malonayl-bis(4-p-chlorophenyl thiosemicarbazide(H4pClMaTS), (b) Co(II), (c) Cu(II), (d) Zn(II) and (e) Sn(II) complexes.  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 792 Table 6. Kinetic parameters using the Coats-Redfern (CR) and Horowitz-Metzger (HM) operated for 1,1-malonayl-bis(4-p-Chloro- phenylthiosemicarbazide) (H4pClMaTS) and its Co(II), Cu(II), Zn(II) and Sn(II) complexes. Parameter Compound Stage Method E (J·mol−1)A (s−1) ΔS (J·mol −1·K−1)ΔH (J·mol−1) ΔG (J·mol−1) R CR 1.79 × 105 1.94 × 1017 8.20 × 101 1.75 × 105 1.35 × 105 0.9997 HM 1.54 × 105 7.15 × 1014 3.54 × 101 1.50 × 105 1.33 × 105 0.9992 I 1st Average 1.66 × 105 9.74 × 1016 5.87 × 101 1.62 × 105 1.34 × 105 0.9995 CR 4.70 × 104 1.40 × 102 –2.08 × 102 4.27 × 104 1.50 × 105 0.9963 HM 6.65 × 104 4.13 × 104 –1.61 × 102 6.22 × 104 1.45 × 105 0.9956 Ia 1st Average 5.67 × 104 2.07 × 104 –1.85 × 102 5.25 × 104 1.48 × 105 0.9960 CR 6.07 × 104 2.32 × 103 –1.86 × 102 5.61 × 104 1.58 × 105 0.9931 HM 6.87 × 104 2.35 × 104 –1.66 × 102 6.42 × 104 1.55 × 105 0.9918 Ib 1st Average 6.47 × 104 1.29 × 104 –1.76 × 102 6.02 × 104 1. 57 × 105 0.9925 CR 4.58 × 104 1.17 × 1047 6.52 × 102 4.54 × 105 1.33 × 105 0.9977 HM 4.65 × 105 1.20 × 1048 6.70 × 102 4.61 × 105 1.30 × 105 0.9982 Ic 1st Average 4.61 × 105 6.57 × 1047 6.62 × 102 4.57 × 105 1.32 × 105 0.9980 CR 2.76 × 1041.68 × 1027 2.72 × 102 2.72 × 105 1.36 × 105 0.9950 HM 2.95 × 1051.77 × 1029 3.11 × 102 2.90 × 105 1. 35 × 105 0.9945 Id 1st Average 2.85 × 105 8.94 × 1028 2.91 × 102 2.81 × 105 1.35 × 105 0.9948 Figure 6. The suggested Octahedral structure of 1,1-malonayl-bis (4-p-chloro- phenylthiosemicarbazide) Metal trihydrate Complex. M = Co(II), Cu(II), Zn(II) and Sn(II). trated to the thermal stability of the complexes. ΔG is positive for reaction for which ΔH is positive and ΔS is negative. The reaction for which ΔG is positive and ΔS is negative considered as unfavorable or non spontane- ous reactions. Reactions are classified as either exother- mic (ΔH < 0) or endothermic (ΔH > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (ΔG < 0) or endergonic (ΔG > 0) on the basis of whether the free energy of the system decreases or increases during the reaction. The thermo- dynamic data obtained with the two methods are in har- mony with each other. The activation energy of All 1,1-Malonayl-bis(4-p-Chlorophenyl) thiosemicarbazide and its Co+2, Cu+2, Zn+2 and Sn+2 complexes is expected to increase in relation with decrease in their radii (Tunali and Ozkar 1993). The smaller size of the ions permits a closer approach of the ligand (H4pClMaTS). Hence, the E value in the first stage for the Zn+2 complex is higher than that for the other Sn+2, Cu+2 and Co+2 complex. The correlation coefficients of the Arrhenius plots of the ther- mal decomposition steps were found to lie in the range 0.9925 to 0.9995 showing a good fit with linear function. It is clear that the thermal decomposition process of all (H4pClMaTS) complexes is non-spontaneous, i.e., the  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 793 complexes are thermally stable. 4. CONCLUSIONS We can concluded from the above discussions on the (H4pClMaTS) ligand and its Co(II), Cu(II), Zn(II) and Sn(II) complexes using the elemental analysis, molar conductivity, IR, Raman, UV, 1HNMR, mass spectra and magnetic properties, as well as TG/DTG, that. Thus the ligand behaves as tridentate in bath saide chelating agent coordinating through azomethine nitrogen, thiolate sul- phur and enolic oxygen as shown in Figure 6. REFERENCES [1] Zhao, Y. (2000) Liquid chromatographic determination of chelates of cobalt(II), copper(II) and iron(II) with 2- thiophe-necarboxald-hyde-4-phenyl-3-thiosemicarbazone. Chromatographia, 51, 231-234. doi:10.1007/BF02490570 [2] Khuhawar, M.Y. and Lanjwani, S.N. (1998) Liquid chromatographic determination of cobalt(II), copper(II) and iron(II) using 2-thiophenaldehyde-4-phenyl-3-thiosemi- carbazone as derivatizing reagent. Talanta, 46, 485-490. doi:10.1016/S0039-9140(97)00213-0 [3] Lunn, G., Phillips, L.R. and Pacula-Cox, C. (1998) Re- versed phase high performance liquid chromatography of 4-(2-pyridyl)-1-piperazinethio-carboxylic acid 2-[1-(pyridyl) ethylidene] hydrazide dihydrochloride, a synthetic thio- semicarbazone with anti-tumour activity. Journal of Chromatography B: Biomedical Sciences and Applica- tions, 708, 217-222. doi:10.1016/S0378-4347(97)00637-3 [4] Hoshi, S., Higashihara, K., Suzuki, M., Sakurada, Y., Sugawara, K., Uto, M. and Akatsuka, K. (1997) Simulta- neous determination of platinum(II) and palladium(II) by reversed phase high-performance liquid chromatography with spectrophotometric detection after collection on and elu-tion from resin coated with dimethylglyoxal bis-(4- phenyl-3-thiosemicarbazone). Talanta, 44, 571-576. doi:10.1016/S0039-9140(96)02064-4 [5] Gismera, M.J., Mendiola, M.A., Procopio, J.R. and Sevilla, M.T. (1999) Copper potentiometric sensors based on copper complexes containing thiohydrazone and thio- semicar-bazone ligands. Analytica Chimica Acta, 385, 143-149. doi:10.1016/S0003-2670(98)00840-X [6] Qu, J.Y., Liu, M. and Liu, K.Z. (1999) Simultaneous de- termination of lead and copper by carbon paste electrodes modified with pyruvaldehyde bis (NN’-dibutyl thiosemi- carbazone). Analytical Letters, 32, 1991-2006. [7] West, D.X., Carlson, C.S., Liberta, A.E. and Scovil, J.P. (1990) The chemical and antifungal properties of the Copper (II) complexes of 2-acetyl-pyrazine 4N-methyl-, 4N-di-methyl-, and 3-hexamethyleneiminyl-thio-semicarba- zone. Transition Metal Chemistry, 15, 383-387. doi:10.1007/BF01177467 [8] West, D.X., Carlson, C.S., Liberta, A.E., Albert, J.N. and Daniel, C.R. (1990) Transtion metal ion complexes of thiosemicarba-zones derived from 2-Acetylpyridine. Tran- sition Metal Chemistry, 15, 341-344. doi:10.1007/BF01177458 [9] Chohan, Z.H. (2009) Metal-based antibacterial and anti- fun-gal sulfonamides: Synthesis, characterization, and bio- logical properties. Transition Metal Chemistry, 34, 153- 161. doi:10.1007/s11243-008-9171-y [10] Liu, M.C., Lin, T.S., Penketh, P. and Sartorelli, A.C. (1995) Synthesis and antitumor activity of 4- and 5-substituted de-rivatives of isoquinoline-1-carboxalde- hyde thiosmicar-bazone. Journal of Medicinal Chemistry, 38, 4234-4243. doi:10.1021/jm00021a012 [11] Liu, M.C., Lin, T.S., Cory, J.G., Cory, A.H. and Sartorelli, A.C. (1996) Synthesis and biological Activity of 3- and 5-amino derivatives of pyridine-2-carboxaldehyde thio- semicar-bazone. Journal of Medicinal Chemistry, 39, 2586-2593. doi:10.1021/jm9600454 [12] Zhu, X., Wang, C., Lu, Z. and Dang, Y. (1997) Synthesis, charac-terization and biological activity of the Schiffbase derived from 3,4-dihydroxybenz-aldehyde and thiosemi- carbazide and its metal complexes with Nickel(II) and Iron(II). Transition Metal Chemistry, 22, 9-13. doi:10.1023/A:1018453316348 [13] Lim, J.K., Mathias, C.J. and Green, A.M. (1997) Mixed- bis(thiosemi-carbazone) ligands for the preparation of copper radiopharmaceuticals: Synthesis and evaluation of tetradentate ligands containing two dissimilar thiosemi- carbazone functions. Journal of Medicinal Chemistry, 40, 132-136. doi:10.1021/jm9605703 [14] Sathisha, M.P., Budagumpi, S., Kulkarni, N.V., Kurdekar, G.S., Revankar, V.K. and Pai, K.S.R. (2010) Synthesis, structure, electro-chemistry and spectral characterization of (d-glucopyra-nose)-4-phenylthiosemicarbazide metal complexes and their antitumor activity against Ehrlich Ascites Carcinoma in Swiss albino mice. European Jour- nal of Medicinal Chemistry, 45, 106-113. doi:10.1016/j.ejmech.2009.09.031 [15] Mostafa, M.M. (2007) Spectroscopic studies of some thio-semicarbazide compounds derived from Girard’s T and P. Spectrochimica Acta Part A: Molecular and Bio- molecular Spectroscopy, 66, 480-486. doi:10.1016/j.saa.2006.02.063 [16] El-Shekeil, A., Al-Yusufy, F., Amin, R.R. and Abdullah, A. (2004) The DC Electrical Conductivity of the Direct Electro-chemically Synthesized Poly (azome-thine-thio- semicarb-zone)-metal complexes. Journal of Inorganic and Organometallic Polymers, 14, 131-148. doi:10.1023/B:JOIP.0000028091.50660.3a [17] Sladjana, B., Novaković, G.A. and Bogdanović, L.V.M. (2005) Transition metal complexes with thiosemicarba- zide-based ligands and the supramolecular arrangement in the Ni(II) complexes of S-methy-lisothiosemicarbazide. Inorganic Chemistry Communications, 8, 9-13. doi:10.1016/j.inoche.2004.10.001 [18] Vukadin, M.L., Slađana, B.N., Bogdanović, G.A., Jokso- vić, M.D. and Mészáros, K. (2007) Transition metal complexes with thio-semicarbazide-based ligands. Part LVI: Nickel(II) complex with 1,3-diphenylpyrazole-4- carboxaldehyde thiosemicarbazone and unusually defor- med coordination geometry. Polyhedron, 26, 3783-3792. doi:10.1016/j.poly.2007.04.012 [19] El-Asmy, A.A., Al-Ansi, T.Y., Amin, R.R., El-Shahat, M.F., Structural studies on cadmium(II), Co(II), Cu(II) Ni(II) and Zn(II) complexes of 1-malonyl bis(4-phenyl- thiosemi-carbazide. Transition Metal Chemistry, 15, 12-  R. R. Amin et al. / Natural Science 3 (2011) 783-794 Copyright © 2011 SciRes. OPEN ACCESS 794 15. [20] El-Asmy, A.A., Al-Ansi, T.Y., Amin, R.R. and Mounir, M. (1990) Spectral, magnetic and electrical properties of 1-succinyl bis (4-phenylthiosemicarbazide) complexes. Polyhedron, 9, 2029-2034. doi:10.1016/S0277-5387(00)84032-2 [21] Amin, R.R. (2010) Chemical and electrochemical prepa- ration for Co(II) complexes of some pyri-dine-2-(1H)- thione-3-cyano-4-(2-methylphenyl)-5,6-ring fused cycloal- kane derivatives. Journal of Phos-Phorus, Sulfur, and Silicon and the Related Elements, 185, 537-543. doi:10.1080/10426500902840861 [22] Amin, R.R. and El-Gemeie, G.E.H. (2001) The direct electro-chemical synthesis of Co(II), Ni(II) and Cu(II) com-plexes of some pyridinethione derivatives. Synthesis Reactivity Inorganic and Nanometal-Organic Chemistry, 31, 431-440. doi:10.1081/SIM-100002230 [23] Refat, M.S., El-Deen, I.M., Amin, R.R. and El-Ghol, S. (2010) Spectroscopic studies and biological evaluation of some transition metal complexes of a novel schiff base li- gands derived from 5-arylazo-salicyladehyde and o-amino phenol. Toxicological & Environmental Chemistry, 92, 1093-1110. doi:10.1080/02772240903252173 [24] Refat, M.S., El-Deen, I.M., Anwer, Z.M. and El-Ghol, S. (2009) Bivalent transition metal complexes of cou- marin-3-ylthio-semicarbazone derivatives: Spectroscopic, antibacterial activity and thermogravimetric studies. Journal of Molecular Structure, 920, 149-162. doi:10.1016/j.molstruc.2008.10.059 [25] Rajesh, K. and Dennis, G.T. (1989) The direct electro- chemical synthesis of metal complexes of 2,2’-dipyridy- lamine. Inorganica Chimica Acta, 157, 51-56. [26] Furniss, B.S., Hannaford, A.J., Smith, P.W.G. and Tatchell, A.R. (1991) Vogel’s textbook of practical organic chemi- stry. Longman Scientific and Technical, John Wiely & Sons, Inc., New York. [27] El-Metwally, N.M., El-Shazly, R.M., Gabr, I.M. and El-Asmy, A.A. (2005) Physical and spectroscopic studies on novel vanadyl complexes of some substituted thio- semi-carbazides. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 61, 1113-1119. doi:10.1016/j.saa.2004.06.027 [28] El-Asmy, A.A., Al-Gammal, O.A., Dena, A.S. and Ghazy, S.E. (2009) Synthesis, characterization, molecular mod- eling and eukaryotic DNA degradation of 1-(3,4-dihydro- xybenzylidene) thiosemicarbazide com-plexes. Journal of Molecular Structure, 934, 9-22. doi:10.1016/j.molstruc.2009.05.039 [29] Refat, M.S. and Killa, H.M.A. (2010) Hammad fetooh, spectro-scopic and thermal characterization of Cu(II), Co(II), Ni(II) and Mn(II) complexes of fluorescent dye 4-N,N-dimethyl-ethanolamine-N-allyl-1,8-naphthalimide (4DMEAN). Journal of Molecular Structure, 983, 122- 132. doi:10.1016/j.molstruc.2010.08.041 [30] Chandra, S. and Kumar, U. (2005) Spectral and magnetic studies on manganese(II), cobalt(II) and nickel(II) com- plexes with Schiff bases. Spectrochimica Acta A, 61, 219-24. doi:10.1016/j.saa.2004.03.036 [31] Hassaneien, M.M., Gabr, I.M., Abdel-Rhman, M.H. and El-Asmy, A.A. (2008) Synthesis and structural investiga- tion of mono- and polynuclear copper complexes of 4-ethyl-1-(pyridin-2-yl) thiosemicarbazide. Spectrochimica Acta Part A, 71, 73-79. doi:10.1016/j.saa.2007.11.009 [32] Lever, A.B.P. (1988) Inorganic electronic spectroscopy. Elsevier Publishing Company, Amsterdam, 318-361.
|