1. Introduction
Nature handles chemical substances and processes remarkably sustainable where the chirality of natural products is dominant contrasting the majority of synthetic technical materials. Neither the reason for the preference of chirality nor the mostly strict preference of one enantiomer in natural products is clarified because the equivalence of both enantiomers concerning achiral, scalar properties is claimed by parity. The exact validity of parity is generally accepted for preparative chemistry [1] [2] [3] [4] . Thus, stereogenic centres generated from achiral starting materials under achiral conditions are expected to form racemates of an exact 1:1 composition of the enantiomers. The theoretically predicted [5] and experimentally verified [6] deviations from parity for weak interactions of elemental particles induced a discussion [7] [8] [9] [10] [11] if there are also very small deviations from parity in chemistry. Attempts of a direct measurement of deviations from the 1:1 composition of racemates formed from achiral material have not been successful; the therefore necessary measurement of extremely small differences of high concentrations seems to be an extraordinarily difficult problem for chemical analytics. Moreover, chiral contaminations as a consequence of contact with the biosphere may cause artifacts such as arbitrary optical induction in the syntheses of racemates or kinetic or chromatographic resolution, such as by traces of bio materials adsorbed at surfaces. Research is now concentrated to indicate small differences of scalar properties of enantiomers with essentially four concepts: Firstly measurements of differences in IR frequencies [12] - [17] and rotational spectra [18] of enantiomers of small molecules, secondly the indication of different IR-CD effects of enantiomers [19] , and thirdly the different coiling of enantiomeric polymers [20] . Any chiral contamination of samples remains the main problem for the latter. Fourthly, a detection by CD spectra (circular dichroism) of simple derivatives of ammonia were suggested [21] . As a consequence, a novel concept would bring about progress in this fundamental discussion.
2. Experimental
2.1. Spectroscopy
IR spectra: Perkin Elmer 1420 Ratio Recording Infrared Spektrometer, FT 1000; UV/Vis spectra: Varian Cary 5000 and Bruins Omega 20; fluorescence spectra: Perkin Elmer FS 3000 (totally corrected); CD spectroscopy: Jasco J810 Spectrapolarimeter, spectral bandwidth 0.5 nm, integration time 0.5 and 1 s, data interval 0.2 nm; NMR spectroscopy: Varian Vnmrs 600 (600 MHz); mass spectrometry: Finnigan MAT 95.
2.2. Chemicals
(1,1'-Biphenyl)-2,2'-diamine (1, RN 1454-80-4), was supplied from Sigma-Aldrich (product No. 727601) and imidazole (RN 288-32-4) from BASF. Perylene-3,4,9,10-tetracarboxylic-3,4-anhydride-9,10-(1-hexylheptylimide) (2) was prepared according to the literature [22] .
2-(1-Hexylheptyl)-9-{2'-[6-(1-hexylheptyl)anthra[2,1,9-def;6,5,10-d'e'f']diisoquinoline-1,3,8,10-tetraone-2-yl]diphenyl-2-yl}anthra[2,1,9-def;6,5,10-d'e'f'] diisoquinoline-1,3,8,10-tetraone (3): Perylene-3,4,9,10-tetracarboxylic-3,4-anhydride-9,10-(1-hexylheptylimide) (2, 212 mg, 369 µmol) [22] and (1,1'-biphenyl)-2,2'-diamine (1, 34 mg, 185 µmol) in imidazole (1 g) were heated with the exclusion of air and moisture (Ar atmosphere) for 5 h at 140˚C, diluted with ethanol after cooling (10 mL), precipitated with 2 m aqueous HCl (20 mL), stirred for 1 h, collected by vacuum filtration and purified by column separation (silica gel, chloroform to remove a forerun and then silica gel, chloroform/ethanol 20:1 for the main fraction). Yield 111 mg (46%) dark red solid, Rf (silica gel, chloroform/ethanol 20:1): 0.29, IR (KBr):
= 2923.6 (m), 2854.5 (m), 1695.5 (m), 1655.2 (s), 1592.0 (s), 1577.0 (m), 1505.7 (w), 1480.6 (w), 1432.9 (w), 1403.9 (m), 1337.8 (s), 1249.9 (m), 1174.2 (w), 1124.7 (w), 1105.9 (w), 964.2 (w), 847.9 (w), 808.5 (m), 744.6 (m), 637.4 cm−1 (w), 1H NMR (600 MHz, CDCl3, 25˚C): δ = 0.82 - 0.92 (m, 12 H, CH3), 1.22 - 1.49 (m, 32 H, CH2), 1.92 - 2.04 (m, 4 H, β-CH2), 2.25 - 2.35 (m, 4 H, α-CH2), 5.22 - 5.24 (m, 2 H, α-CH), 7.24 - 7.25 (m, 2 H, arom. CH), 7.47 - 7.50 (m, 2 H, arom. CH), 7.69 - 72 (m, 2 H, arom. CH), 7.79 - 7.80 (m, 2 H, arom. CH), 8.13 - 8.51 ppm (m, 16 H, arom. CH), 13C NMR (151 MHz, CDCl3, 25˚C): δ = 14.3, 22.9, 27.2, 29.5, 32.0, 2.1, 32.5, 32.7, 55.1, 122.7, 122.9, 123.0, 123.3, 123.4, 124.0, 124.3, 126.1, 126.5, 128.9, 129.0, 129.2, 129.3, 129.6, 130.9, 131.8, 133.5, 133.8, 134.1, 134.3, 134.7, 134.8, 138.2, 162.8, 163.8, 164.2 ppm, UV/Vis (CHCl3): λmax (ε) = 262 (63,400), 461 (37,400), 492 (102,900), 533 nm (152,200 L・mol−1・cm−1), fluorescence (CHCl3): λmax = 539, 582 nm, fluorescence quantum yield (CHCl3, λex = 490 nm, E490nm = 0.240 cm−1, reference [23] : 2,9-Bis-(1-hexylheptyl)anthra[2,1,9-def;6,5,10-d’e’f’]diisoquinoline-1,3,8,10-tetraone, RN 110590-84-6 [24] , with Φ = 1.00): 1.00, MS (DEI+/70 eV): m/z (%): 1294 (4) [M+], 931 (39), 182 (38), 97 (42), 83 (61), 69 (100), 55 (91), 44 (24). C86H78N4O8 (1295.6): calcd. C 79.73, H 6.07, N 4.32; found C 79.26, H 6.26, N 4.26.
3. Results and Discussion
The detection of an utmost only very small enantiomeric excess in essentially racemic materials as a consequence of a possible chemical violation of parity (PV) requires an analytical signal amplification. We applied optical circular dichroism (CD) [25] as the difference of light absorptivity between left and right circularly polarized light for the detection of a chiral fraction where the effect would be completely compensated for an exact 1:1 racemate. Chiral substituents or chiral media generally induce only very weak CD effects in achiral, flat chromophores such as the perylene dyes; this makes CD spectroscopy of such dyes insensitive and robust concerning traces of chiral contaminants of biological origin. However, very strong CD effects were induced by exciton interactions of adjacent chromophores in chiral arrangements in dyads so that the required high signal amplifications are obtained. Furthermore, special care has to be taken for the exclusion of other possible artifacts such as arbitrary kinetic or chromatographic partial resolution of racemates in chemical synthesis and workup of such chiral arrangements. For this reason, we applied permanently fast racemising [26] [27] [28] [29] axially chiral 2,2'-substituted biphenyls [30] [31] with chiral half-lifes in the order of minutes [32] [33] [34] [35] [36] .
Perylene biscarboximides [37] [38] with the solubilizing swallow-tail N-substituent 1-hexylheptyl [24] [37] were used as the required chromophores for the preparation of the target compound, because of only a single electronic transition [39] in the visible polarized along the N-N-connection line [40] , the strong fluorescence [23] and high chemical persistency [37] . Thus, we condensed [41] 2,2’-diaminobiphenyl (1) with the anhydride carboximide 2 [22] to form the racemic bichromophore (P)-3 and (M)-3; see Scheme 1.

Scheme 1. Synthesis of 3.
The achiral chromophore of perylenebiscarboximides as a component in 3 such as in the soluble derivative 4 (see Scheme 2, top) exhibits a strongly structured intense UV/Vis- absorption with a maximum at 526 nm and a molar absorptivity of 88,000 L・mol・cm−1 in the maximum [42] as is indicated in Figure 1, left, bottom. We sterically locked the chirality in the biphenyl dyad 3 with methyl groups to obtain the pure, stable atrop enantiomers (P)-5 and (M)-5 [43] where exciton effects of the adjacent chromophores both influence the UV/Vis absorption spectra (see Figure 1, left, middle; the spectrum of (P)-5 is identical) and induce very strong CD effects of absolute ε values of about 500 (see Figure 1, left, top); these high exciton-induced CD effects were used as an amplifier for the detection of a chiral fraction in 3. One can expect similarly high CD effects in the unlocked, demethylated 3 in the same spectral region because of a similar arrangement of chromophores (the electronic effect of the methyl groups in 5 on the chromophore are estimated to be unimportant; compare [43] [44] ); however, a fast racemization proceeds to an equilibrium because of the low torsion barrier in the unlocked 3; this excludes artefacts such as by arbitrary chromatographic resolution at chiral surfaces. There will be a compensation of CD effects of (P)-3 and (M)-3 in the racemate so that only an enantiomeric excess will be detected in the CD spectrum of such a dynamic racemate. We found very weak concentration-dependent CD signals of 3 in the expected spectral region where strong
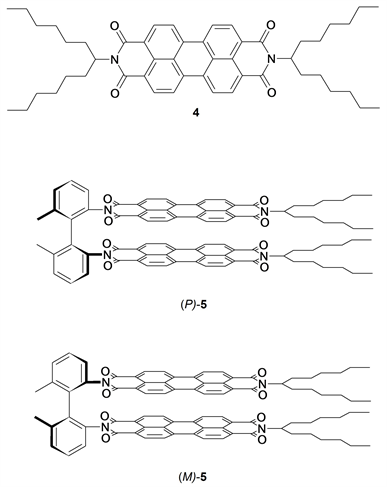
Scheme 2. The achiral dye S-13 (4) and the sterically locked, stable chiral atropisomeric dyes (P)-5 and (M)-5, for comparing CD effects.
![]()
Figure 1. Left from bottom to top: UV/Vis spectra of 4 (blue), (M)-5 (red), and the CD spectra of (P)-5 (orange) and (M)-5 (turquoise) for indicating the CD effect of perylenes in sterically locked biphenyls. Right from bottom to top: CD spectra of 4 (blue, Emax/1 cm = 0.64), 3 (red, Emax/1 cm = 2.14), 4 (turquoise, more concentrated: Emax/1 cm = 2.36), 3 (orange, more concentrated: Emax/1 cm = 2.53) indicating a very small enantiomeric excess for (M)-3. All spectra were recorded in chloroform.
signals of the pure enantiomers of 5 were found. These were attributed to a very small enantiomeric excess of the (M)-3 enantiomer where the concentration dependence may be taken as an indicator for a dye specific effect (a further increase of the concentration and the optical density E, respectively, is problematic because of the strong light absorption). Controlling the proper function of the spectrometer, we recorded the spectra of the achiral and in the same spectral region analogically absorbing 4 at similar optical densities and did not get comparably intense CD signals. As a consequence, the CD spectra of 3 can be taken as an indicator for a very small enantiomeric excess of (M)-3.
4. Conclusion
A fast racemizing ensemble of enantiomers may be a useful tool for the detection of a spontaneous formation of an enantiomeric excess where atropisomeric biphenyls are of special interest because the rate of racemisation can be efficiently controlled by steric effects of substituents. Amplifying the detection sensitivity of enantiomeric excesses is necessary because of the small expected effects. Optical methods, preferentially the measurement of strong CD effects induced by exciton interactions of adjacent chromophores, are attractive because of high sensitivity. The investigation of a perylene dyad of biphenyl indicated a very small enantiomeric excess of the (M) enantiomer. One can speculate about the origin of the preference. The well-known parity-violating (PV) asymmetry for weak interactions seems to be very small concerning chemistry; the present study indicates a much larger effect where the order of magnitude might become important for living organisms. This may find its counterpart in the general clear preference each for one individual enantiomer in the biosphere. Finally, exciton interactions of larger chromophores such as 4 in racemizing systems may be an attractive, efficient and sensitive tool for the investigation of chiral effects and search for other examples for PV.
Acknowledgements
This work is supported by the Fonds der chemischen Industrie and the CIPSM Cluster in Munich.