Sepsis Associated Encephalopathy Predicts Poor Outcome among Acute Supratentorial Intracerebral Hemorrhage with Coma ()
1. Introduction
Sepsis is defined as a life-threatening organ dysfunction due to a dysregulated host response to infection [1] . The consensus definition of sepsis requires proven infection, at least one organ failure, and signs that meet two or more criteria for the SIRS [2] [3] [4] [5] . Importantly, sepsis associated encephalopathy (SAE) is the most common type of sepsis that is seen in the ICUs. The incidence of SAE was about 70% of septic patients [6] . The mortality of sepsis patients with primary stroke was 56.5% [7] , especially those who had SAE; its mortality is closer to 63.0% [8] . Therefore, SAE could be identified as a significant risk factor for increased mortality. Acute supratentorial intracerebral hemorrhage (SICH) is a common cause of coma, with a 28-day mortality rate of 43.0% [9] [10] . Recently, the incidence of ICH patients who are at high risk for systemic infection has increased [11] [12] [13] and thus comprehensive treatment strategies are required to treat infection diseases. In particularly, the number of patients who are managing stroke and also suffer from SAE has been increasing, and the mortality rate was over half of patients [7] [8] , suggesting that the prognosis of stroke with sepsis/SAE may be worse. However, there are few special reports discussing predictors that affect the neurological outcome of SICH in patients with SAE. We sought to determine whether the presence of SAE among acute SICH with coma would predict a poor outcome.
2. Methods
2.1. Study Settings
This was a retrospectively study of all registered an adult ICU of university teaching hospital in China between June, 2013 and July, 2015. The study was approved by the Ethical Committee on Clinical Research of Shuyang People’s Hospital, because the study involved only a review of records obtained as a part of routine medical care, without patient consent was required.
2.2. Patients Identified
Based on the International Statistical Classification of Diseases 10th Revision (ICD-10) by WHO in 1994 discharged, we identified those patients who had acute spontaneous supratentorial ICH with coma (code I61.9, R40.2) in a consecutive 2-years period. A total of 447 consecutive patients with ICH with coma were recruited. We excluded infratentorial ICH (n = 57), intraventricular (n = 5), and ICH volume recorded unknown (n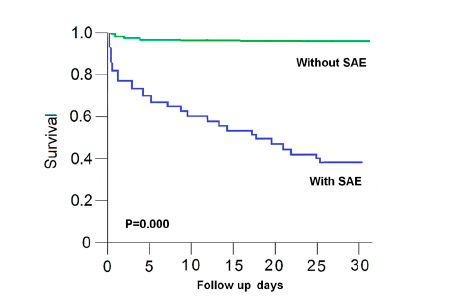 =
=  6). Finally, 379 SICH patients with coma were included in our study.
6). Finally, 379 SICH patients with coma were included in our study.
In this study, sepsis was diagnosed using clinical indicators of suspected infection and concurrent acute organ failure [1] [2] [3] [4] [5] , adapting Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) criteria [1] . For SAE, we used the following diagnostic criteria: 1) acute brain dysfunction is related to sepsis, including an inflammatory response processes and at least exhibiting a sepsis-related organ failure [1] [2] [3] [4] [5] ; and 2) a lower Glasgow Coma Scale (GCS) score [14] that could not be fully explained by isolated ICH. The exclusion criteria were used for SAE: 1) with evidence of meningitis/encephalitis; 2) with evidence of non-septic encephalopathies; and 3) with evidence of organ dysfunction due to the effects of sedatives or other medications.
2.3. Clinical Assessment
All clinical parameters was measured based on data recorded in the initial 24 h of admission to ICU and the sepsis-related Sequential Organ Failure Assessment (SOFA) score was assessed later 5 days.
All patients underwent an initial brain CT scan on admission and at least one repeat brain CT scan after coma onset. We analyzed the CT data that were collected at the closest in time following onset. The hematoma volumes on admission and closest in time of rebleeding were measured and using the standard ABC/2 formula (where A is the longest diameter of the hematoma, B is the widest diameter of the hematoma, and C is the layer thickness of the hematoma) [15] .
Infection was assessed using clinical two or more systemic inflammatory response syndrome (SIRS) criteria: 1) temperature greater than 38˚C or less than 36˚C; 2) heart rate greater than 90 beats per minute; 3) tachypnea > 20 respirations per minute or Pco2 < 32 mmHg; 4) white blood cell count greater than 12.0 × 109/L or less than 4.0 × 109/L, or more than 10% band forms [3] .
Sepsis-related organ failure was diagnosed by a sepsis-related Sequential Organ Failure Assessment (SOFA) score (range 0 - 4, with higher scores indicating more severe organ failure) ≥ 2 for a particular organ after the onset of infection [16] . The following were considered to be organ failure: [16] brain―GCS < 13 score; respiratory―bilateral infiltrates on chest thorax radiograph and arterial oxygen pressure/fraction of inspired oxygen ratio (PaO2/FiO2) ≤ 300 or the need for supplemental oxygen to maintain oxygen saturation > 90% (excluding the prior need for oxygen); circulation―hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 65 mmHg or decrease > 40 mmHg in the systolic pressure); liver―total serum bilirubin > 33 uml/L; renal―creatinine > 171 uml/L; blood―platelet count ≤ 100 × 109/L, glucose > 27.8 mmol/L or <2.8 mmol/L, or sodium > 160 mmol/L or <110 mmol/L.
The following data in SICH patient with coma were also collected to examine the relationships between clinical outcome, including age, sex, ICU days, underlying disease, hematoma location, hemorrhage growth, hematoma volume, accompanying intraventricular hemorrhage, acute physiology and chronic health evaluation II (APACHE II) score, initial National Institutes of Health Stroke Scale (NIHSS) score. We recorded the onset-to coma time, the length of ICU stay, and outcomes at 30 days of follow-up.
2.4. Related Definition
Significant intracerebral hemorrhage growth was defined as hematoma expansion of greater than one-third on repeat CT [17] or hematoma enlargment lead a brain herniation. Midline shift was measured as described in the literature [18] [19] . Cisterns were recorded as normal, compressed, or absent, based on Marshall Scale criteria [20] . Brain herniation has been divided in to uncal and central types [21] . Central herniation referred to the ascending reticular activating system (ARAS) compressed in the central diencephalon, radiological with midline shift and perimes-encephalic cisterns compressed, and clinically with stupor or coma. Uncal herniation was more likely to have the unilateral uncal gyrus downward displacement and was compressed the midbrain and ARAS than bilateral, radiological with midline shift and the third nerve compressed, and clinically with coma, pupil asymmetry of >2 mm and loss of reactivity to light.
2.5. Statistical Methods
The results in each group were expressed as mean ± standard deviation (SD) or medians (IQR), and n (%) for qualitative values. Continuous variables were compared using the t test. Chi-squared tests and Pearson’s correlation coefficients were used to explore the relationships between baseline variables. Multivariate-adjusted risk ratios (RR) and 95% confidence intervals (CIs) were estimated with the use of the logistic-regression model if they were significant in the univariate analysis, or Cox proportional hazards model to examine severe sepsis baseline status and determine whether the variables played a role in the risk of death events. Survival analysis was performed using the Kaplan-Meier curve method. There was significantly difference between the groups if the P-value was < 0.05. Statistical calculations were performed using a proprietary, computerized statistics package (SPSS 10.0).
3. Results
Of 379 patients included into the study, mean age was 61.5 ± 10.6 years, 62.0% were male, 231 (60.9%) had a prehospital coma (PC) events, and 148 (39.1%) had a nosocomal coma (NC) events. Of the SICH, 58.9% had a striato-capsula hemorrhage. For SICH underlying diseases and more baseline characteristics, see Table 1.
Among 379 SICH with coma, 245 (64.6%) had SAE events. The characteristics of SICH patients with SAE and no SAE are shown in Table 2. In patients with SAE, the most frequent SIRS criterion that was met was at a threshold of four SIRS criteria (51.0%), whereas at a threshold of two SIRS criteria only 23.1%. We found that there was significantly difference in sepsis events (100.0% vs 5.2%, p = 0.000), temperature (38.1 ± 1.2 vs 37.1 ± 1.0, P = 0.000), pulse rate (96.0 ± 15.8 vs 85.0 ± 15.5, P = 0.000), elevated WBC (14.8 ± 5.5 vs 12.2 ± 6.2, P = 0.000) in addition to respiratory rate (24.9 ± 5.8 vs 23.8 ± 6.4, P = 0.096) between the two groups. Infection was higher in patients with SAE than in patients without SAE (100% vs 35.8%, P < 0.001). In patients with SAE, the commonest infection focal was mainly from respiratory infection (58.4%). while hospital-acquired infection was present in 48.2% and community-acquired infection was present in
![]()
Table 1. Baseline characteristics in supratentorial intracerebral hemorrhage patients with coma (n = 379).
Abbreviation: NC, nosocomal coma; PC, prehospital coma; OCT, onset-to-coma time; GCS, Glasgow coma scale; SBP, systolic blood pressure; DBP, diastolic blood pressure.
51.8%. Most of the patients were performed bacteriological examination, and blood cultures were microbiologically confirmed in minority of the clinical sepsis (35.1%). In patients with SAE, the commonest subsequent organ failure was nosocomal brain failure (60.4%). The patients with SAE were significantly more likely to present with higher SOFA score (4.1 ± 1.4 vs 3.3 ± 0.7, P = 0.000) and higher number of organ failure (1.2 ± 0.9 vs 0.1 ± 0.3, P = 0.000) than those no SAE. In correlate analysis, the number of organ failure in SICH patients with SAE were positively correlated with the number of SIRS’ criteria from 0 - 4 (r = 0.671, P = 0.000), and also was positively correlated with death (r = 0.490, P = 0.000).
On those univariate analyses of patients with SAE and no SAE after SICH are summaried in Table 3. We found that in addition to no difference in sex and
![]()
Table 2. The characteristics in SICH patients with SAE and no SAE (n = 379).
Abbreviation: SAE, sepsis associated encephalopathy; SICH, supratentorial intracerebral hemorrhage; SIRS, systemic inflammatory response syndrome; SOFA, sequential organ failure assessment.
age, APACHE II score, SOFA score, sepsis events, acute respiratory failure, nosocomal brain failure, septic shock, acute renal failure, hypernatremia, acute seizure, midline shift, basal cisterns compressed, vasogenic edema, white matter lesions, central herniation, uncal hemiation, and hematoma volume was significantly associated with differences between the groups (all P < 0.05). However, in Cox multivariate logistic analysis, only the SAE (RR, 4.4; 95% CI, 2.296 - 8.422; P = 0.000) was significantly related to risk on death in SICH patient (Table 4).
During 30 days follow-up, survival data were available for our patients with SAE or no SAE. The survival rate was higher among SICH patients without SAE
![]()
Table 3. Univariate analyses of SICH patients with SAE and no SAE (n = 379).
Abbreviation: SAE, sepsis associated encephalopathy; SICH, supratentorial intracerebral hemorrhage; APACHEII, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment.
![]()
Table 4. Cox multivariate analysis of the association of outcome for SICH patients with coma (n = 379).
Abbreviation: SAE, sepsis associated encephalopathy; SICH, supratentorial intracerebral hemorrhage; SOFA, sequential organ failure assessment.
than among those with SAE. The respective mortality rates among SICH patients with SAE were significantly higher (60.8% vs 11.2%) at 30 days. Kaplan-Meier survival curves show that patients with SAE had significantly worse survival than patients without SAE (risk ratio = 6.8; 95% CI, 4.630 - 13.16; P = 0.000). (Figure 1)
4. Discussion
Both SAE and SICH are a significant cause of coma and death throughout the
![]()
Figure 1. Kaplan-Meier survival curves for SICH patients with sepsis and no sepsis. Kaplan-Meier survival curves show that SICH patients with sepsis had significantly worse survival than patients no sepsis during the 30 days follow-up (risk ratio = 6.8; 95% CI, 4.630 - 13.16; P = 0.000).
world. Previous studies demonstrated that a decreased GCS, increased ICH volume, and presence of intraventricular hemorrhage were related to neurological deterioration and the high rate of poor outcomes in ICH patients [22] [23] [24] [25] . Current studies emphasized that neurological deterioration due to hermatoma enlargement, including early hematoma growth, was poor outcome and increased mortality factors in SICH [16] [26] [27] . Our study showed that the mean volume of hematomas in the SAE group was significantly larger than those who did no. This suggests that hematoma volume or hermatoma growth was related to poor outcome in SICH patient with coma. However, in our study, 245 (64.6%) SICH patients with SAE were diagnosed according to the criteria for SAE. Therefore, our data suggests that there was a high prevalence of SAE in patients with SICH with coma.
Several studies have demonstrated that the prevalence of infection among patients with ICH is high [11] [12] [13] , and that the sepsis in stroke patients did not be infrequency [8] [28] . Our study showed that the patients who had infection and organ failure, a threshold meeting two or more SIRS criteria occurred in 64.6% (245/379) of SICH patients with coma, supporting these acute SICH patients with coma had SAE events.
On proportion analysis, mortality increased significantly with each additional SIRS criterion from 2 to 4, without any transitional increase in risk at a threshold of one SIRS criteria. Moreover, our study confirmed that the mortality of SAE among SICH patients who met four SIRS criteria was 20% higher than that those who met 2 - 3 SIRS criteria, and the number of SIRS criterion that SICH patients met were positively correlated with the number of organ failure. This showed that the higher grades of SIRS and the scores of SOFA, the more poor of the short-term outcome will be.
Brain herniation is a common form of acute brain failure. Our data showed that such organ failure due to sepsis was mostly focused on brain failure. The rates of uncal herniation events in SICH patients with SAE were significantly higher than in those who did not. Brain herniation had usually resulted from supratentorial mass effect [18] [24] , but current study demonstrated that vasogenic edema was significantly higher in SICH patients with SAE than those who did not. The development of vasogenic cerebral edema is related to the inflammatory mechanism [29] [30] [31] . Furthermore, there is evidence of blood-brain barrier dysfunction in both patient and rodent models of sepsis [32] . Our data showed that late brain failure were present in 60.4% sepsis after acute SICH. According to literature records, 70% of sepsis might develop brain dysfunction [6] [33] , suggesting that our majority of SICH patients with sepsis may present with the component of SAE. Moreover, a Cox multivariate risk analysis is also confirmed that SAE and uncal herniation may be more likely to relate to worse outcome in SICH patient with coma.
The above findings confirmed that the SAE has impaired the outcome and mortality of SICH patients with coma. Our findings suggest that three aspects are possible interpretations. One hand, the mortality of SAE is high, which has been confirmed [7] [8] . Second hand, the SAE patients with a GCS score < 8 had a poor outcome and a high risk of death, which was also confirmed by the previous study [8] . In addition, other factors such as high SOFA score, acute renal failure, acute respiratory failure, vesogenicedeam, and greater hematoma volume may affect jointly the host response and increase the risk of the death, which is confirmed by current Cox multivariate risk analysis. However, this study’s findings showed that high prevalence of SAE and its multiple organ failure was more likely to develop a poor outcome among SICH patients with coma. Moreover, previous studies suggested that a high mortality rate in sepsis is almost always due to multiple organ failure [34] . Therefore, knowing the above cases of sepsis after patients with SICH may help identify clinical therapeutic targets, and assist in decision making for SICH patients with sepsis who develop coma.
Limitations: some limitations have to be considered in our study. First, restriction to SICH patients with coma in this set may have to be created an overestimation of incidence for SAE. However, high rate of 70% in patients with SAE has been reported [6] . Second, some patients had not microbiologically examined or only confirmed by the sputum culture, this may be the reason for the low positive rate of microbiologic proof of infection, but the blood culture negative sepsis was not uncommon [35] [36] . Furthermore, the consensus has been formed that sepsis diagnostic criteria requires proven infection and organ failure, and signs that meet two or more criteria for the SIRS [2] [3] [4] [5] [36] . Therefore, we believe, high prevalence of SAE events in SICH patients with coma in current study should not be overestimated. In addition, about 70% of septic patients were associated with an encephalopathy [6] , while subcortical white matter ischemic lesions and microinfarctions have been confirmed [4] . Thus, a brain MRI for SICH patients with sepsis and with brain lesions is very important, however, the scan of MR imaging in this study was absent.
Conclusion: SAE is a frequent complication of SICH, which greatly increased risk of death in SICH patients with coma. It is research novelty that SAE may be pivotal in the pathogenesis of SICH patients with coma, which should be received attention in the field of SICH Care.
Acknowledgements
This work is supported by a grant from the Medical Research Council, Shuyang Hospital, Xuzhou Medical University, China.