Catalytic Properties of Various Oxides and Mesoporous Materials Containing Niobium and Sulfate Ions, in the Oxidation Reaction of 1-Octanol ()
1. Introduction
Esters are common in organic chemistry, and their synthesis is one of the most fundamental transformations. The reaction is usually performed by the coupling of a carboxylic acid or its derivative with an alcohol [1] in the presence of an acid catalyst. Recently, catalytic methods such as condensation of an equimolar amount of carboxylic acid and alcohol [2] and cross Tishenko esterification of aldehydes with alcohols [3] have been developed. However, the development of novel catalytic systems for direct ester synthesis from stable and readily available substrates still remains a major challenge. Considering that alcohols are less corrosive and more accessible than acids or aldehydes, the direct aerobic oxidation of alcohols to esters is an important alternative which has been accomplished with several homogeneous and heterogeneous catalytic systems [4] [5] .
In our previous work [6] , we prepared catalysts that showed some interesting redox properties alongside their intended acidic nature. During one specific experiment where we performed the etherification of 2-naphtol (1) with 1-octanol (6) in Scheme 1, an interesting side-product octyl octanoate (8) was formed with naphtofuran (11).
This observation showed us an interesting opportunity that could potentially lead to the establishment of a new method for ester synthesis by reacting a primary alcohol with our catalyst. The next step was to establish this new method and to understand the mechanism of this potentially useful reaction. For this purpose, we tested our catalysts with the primary alcohol 1-octanol (6) due to its higher boiling temperature (195˚C), since this transformation requires high temperatures.
This reaction was done by a straight forward experimental procedure, where a known amount of catalyst was added to 1-octanol under stirring and heating. Optimizations of the reaction parameters (time, temperature and catalyst loading) were also done.
During the catalytic tests realized on this reaction, several products were obtained, each one resulting from a different aspect of the catalyst’s nature; for example, the dioctyl ether (7) was a result of the catalyst’s surface acidity, and other products (8), (9) and (10) were a result of the redox properties of our catalysts. Each catalyst had different selectivity which will be discussed later.
2. Experimental
2.1. Materials and Equipment
All information and acidity determination method are detailed in our previous article [6] . The test for peroxide intermediates was done using Peroxide 25 and 100 test sticks purchased from MACHEREY-NAGEL.
2.2. GC Technique
GC analyses were performed on a GC-2025 Gas Chromatograph (Shimadzu) equipped with a ZB-5-MS (column 30.0 m, 0.25 mm internal diameter, 0.25 μm
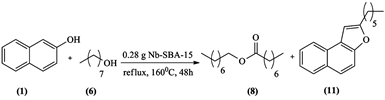
Scheme 1. Etherification of 2-naphtol with 1-octanol.
film thickness, 5% Polysilarylene -95% Poly dimethyl siloxane) and with a Flame Ionization Detector. The GC program applied throughout all the analysis to obtain quantitative information on the oxidation reaction is as follows: the carrier gas was N2, at a flow rate of 1.27 mL/min. Column temperature was initially 70˚C for 2 min, then gradually increased to 280˚C at 10˚C/min and finally kept at 280˚C for 15 min. Throughout the analysis, the injector and detector temperatures were kept constant at 250˚C and 280˚C, respectively. The analysis was performed on split mode with a split ratio of 40.
2.3. Catalysts Preparations
Several solid acid catalysts were prepared, including microporous oxides (ZrO2, TiO2, HfO2) and siliceous mesoporous materials (MCM-41 and SBA-15). The detailed preparations and morphological properties of these solid catalysts is given in our previous article [6] . Both morphological properties (surface area and nature of phase) of these prepared catalysts will be mentioned again here in Table 1 taken from our previous work [6] .
2.4. Catalytic Test
The oxidation reaction of 1-octanol, obtained products and reaction conditions used are shown in Scheme 2.
General procedure for 1-octanol oxidation: The oxidation of 1-octanol (6) was done in a 25 mL double necked round bottom flask equipped with a condenser and a thermometer. 1.3 g (10 mmol) of 1-octanol were added to 0.2 g of catalyst (15 wt∙%). The mixture was stirred (300 - 400 rpm) at 160˚C for 36 h, then cooled down to room temperature, filtered and washed with DCM. The filtrate was concentrated to give the crude product (a dark colored oil).
After each experiment the crude was weighed, and an average of 5 - 10 wt∙% loss of mass was recorded (due to 1-octanol evaporation during the reaction). 1H
![]()
Table 1. Surface area and phase of the prepared catalysts.
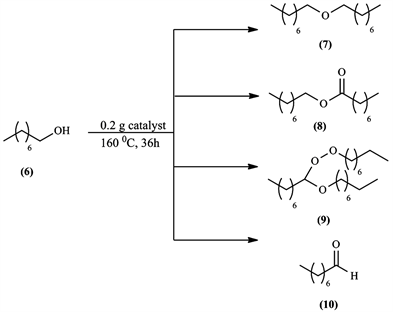
Scheme 2. Products resulting from the reaction of 1-octanol.
NMR aliquot was made from crude to check the conversion of the starting material and product composition, these roughly estimated results were compared to the more accurate results obtained from GC analysis, and a difference of approximately 5% - 10% as average was seen between the two results in each experiment.
The progress of the reaction was monitored by TLC, using as eluent cyclohexane and ethyl acetate 95/5 in volume. The crude was separated on silica gel column chromatography using the same eluent, and the fractions obtained were also a mixture of products (due to similar Rf values) so a further separation on a preparative TLC glass plate (SiO2, cyclohexane/ethyl acetate = 95/5) was necessary to obtain pure fractions of the products for structure identification. This is in case of peroxyacetal (9) because it wasn’t commercially available.
The structure of products obtained was confirmed by 1H NMR, 13C NMR and MS analysis.
2.5. NMR Product Estimation Method
The reaction was stopped and an aliquot of the organic phase was analyzed by 1H NMR without further treatment (the sample was filtered on a micro syringe filter, to remove any catalyst particles). All the compounds of the reaction mixture were attributed by their characteristic chemical shifts. The percentages of compounds were calculated by the ratios of the integration of one proton of the considered compound on the sum of integration of all the compounds (normalized to one proton). By this method was deduced the conversion (or transformation rate).
2.6. Analysis of Products by Gas Chromatography
The determination of 1-octanol conversion and product selectivity was obtained by using multiple point internal standard calibration method for the GC analysis, eicosan was used as an internal standard. The identification of products was made by comparison of retention times with pure samples and confirmed with mass spectroscopy.
Each analysis contains the IS (internal standard) whose concentration is kept constant and the compound of interest. The results were plotted with the ratio of the area of the compound to the area of the internal standard on the y-axis and the ratio of the concentration of the compound to the concentration of internal standard on the x-axis. This data was fitted to produce a calibration curve.
Products retention times and calibration curve equation are shown in Table 2.
After the reaction was stopped and processed, a sample of the crude product was subjected to GC analysis. To prepare this sample, a weighed amount of the crude was added to 10 mL of eicosan solution (C = 1.05 mg/mL) and the volume was completed to 20 mL with CH2Cl2.
Therefore octanol conversion and product selectivity were calculated using the following equations:
n0 (octanol): the initial number of moles of 1-octanol;
ntf (octanol): the final number of moles of 1-octanol (the remaining unreacted quantity);
ni(t): is the number of moles of product i formed at the end of the reaction.
Characterization of the products:
1-(octyloxy)octane (7)
1H NMR(300 MHz, CDCl3, δ ppm): 3.39 (t, 4H), 1.57 (m, 4H), 1.27 (m, 20H), 0.88 (t, 6H). 13C NMR(75 MHz, CDCl3): 72.3 (2 CH2), 33.45 (2 CH2), 32.65 (2 CH2), 31.98 (2 CH2), 30.26 (2 CH2), 25.35 (2 CH2), 21.76 (2 CH2), 16.12 (2 CH3). HRMS-ESI: m/z [MNa]+ calculated for [C16H34O]+: 242.2618 found: 242.2645.
![]()
Table 2. Retention times and regression line equations of pure product samples.
Colorless oil Rf: 0.72 (aluminum sheets coated with silica gel Merck 60 F254 0.25 mm, cyclohexane/ethyl acetate: 95/5).
Octyl octanoate (8)
1H NMR(300 MHz, CDCl3, δ ppm): 4.05 (t, 2H), 2.29 (t, 2H), 1.59 (m, 4H), 1.28 (m, 18H), 0.88 (t, 6H). 13C NMR(75 MHz, CDCl3): 173.97 (Cq), 64.42 (CH2), 34.46, 31.88, 31.77, 29.31, 29.28, 29.21, 29.04, 28.76, 26.04, 25.11 (C10H20), 22.68 (2 CH2), 14.12 (2 CH3). HRMS-ESI: m/z [MNa]+ calculated for [C16H32O2]+: 256.2408 found: 256.2601. Colorless oil, Rf: 0.68 (aluminum sheets coated with silica gel Merck 60 F254 0.25 mm, cyclohexane/ethyl acetate: 95/5).
1-(octyloxy)-1-(octylperoxy)octane (9)
1H NMR(300 MHz, CDCl3, δ ppm): 4.44 (t, 1H), 3.40 - 3.53 (m, 2H), 1.55 (m, 6H), 1.26 (m, 32H), 0.87 (t, 9H). 13C NMR(75 MHz, CDCl3): 103.25 (CH), 65.50 (CH2), 63.07 (CH2), 31.98, 30.06, 29.58, 29.43, 26.43, 22.79 (C18H36). HRMS-ESI: m/z [MNa]+ calculated for [C16H32O2]+: 386.3803 found: 386.3901. Colorless oil, Rf: 0.6 (aluminum sheets coated with silica gel Merck 60 F254 0.25 mm, cyclohexane/ethyl acetate: 95/5).
3. Results and Discussions
3.1. Reaction Conditions Optimizations
Reaction conditions (time, temperature and catalyst loading) were optimized. For this purpose, one specific catalyst was chosen, the
(1 M). These optimal conditions were applied to all the following catalytic tests.
3.2. Optimization of Catalyst Loading
Optimal catalyst loading was determined for higher 1-octanol conversion rates. Oxidation reaction of 1-octanol was done following the general procedure detailed in (Section 2.6). The obtained results are shown in Figure 1.
As shown in Figure 1, the optimal catalyst loading was 15 wt∙%, above this value no significant increase in conversion rate was observed.
3.2.1. Optimization of Reaction Temperature
Optimal reaction temperature was determined for maximum conversion rate of 1-octanol. The results are shown in Figure 2.
![]()
Figure 1. Effect of catalyst amount on 1-octanol conversion.
![]()
Figure 2. Effect of reaction temperature on 1-octanol conversion.
As shown in Figure 2, no reaction was detected at temperatures below 140˚C. As the temperature increases the conversion increases accordingly, but at temperatures 175˚C and above larger amount of 1-octanol was lost due to evaporation. This was proven by the great loss of mass in the final crude product, up to 40 - 50 wt∙%. As observed on the curve, above 160˚C the effect is small and not useful. For this reason the optimal temperature for 1-octanol conversion was chosen at 160˚C, at which only 5 - 10 wt∙% of crude was lost due to evaporation.
3.2.2. Optimization of Reaction Time
Optimal reaction time (duration) was determined for maximum 1-octanol conversion. Results obtained are shown in Figure 3.
As shown in Figure 3, conversion rates increased with time until it reached a maximum value at 36h, after which no significant increase in 1-octanol conversion was observed. This is indicating a strong relation between the reaction and the relative concentration of octanol and may be explain by the requirement of several molecules of octanol in the key step.
Finally it was determined that the optimal conditions for this reaction were; catalyst loading 15 wt∙%, temperature of 160˚C and a duration of 36 h.
3.3. Catalytic Evaluation of MCM-41 Catalysts
MCM-41 catalysts were tested with the oxidation reaction of 1-octanol. The obtained results are shown in Table 3.
MCM-41 catalysts showed some interesting catalytic properties, different than expected. Earlier observations in the 2-naphtol etherification [6] with MCM-41 catalysts showed redox properties only when impregnated with niobium, and weak acidic properties when sulfated. Instead in the 1-octanol oxidation, even when sulfated the support showed both acidic and redox properties. The acidic character was dominant observed through higher dioctyl ether (7) selectivity. Even MCM-41 had redox properties with very poor conversions. Also when the
![]()
Figure 3. Effect of reaction time on 1-octanol conversions.
![]()
Table 3. Catalytic evaluation of MCM-41 catalysts.
MCM-41 was in-situ impregnated with niobium, low conversions were obtained but interestingly high selectivity for the octyl octanoate (8).
3.4. Catalytic Evaluation of SBA-15 Catalysts
SBA-15 catalysts were tested with the oxidation reaction of 1-octanol. The obtained results are shown in Table 4.
SBA-15 catalysts showed a slightly better catalytic activity compared to MCM-41. When sulfated the SBA-15 showed both greater acidic than redox properties (higher dioctyl ether (7) selectivity). It showed like MCM-41 poor redox properties with very low conversions. When impregnated with niobium it showed higher redox properties with high selectivity for octyl octanoate (8), especially when in-situ impregnated.
MCM-41 materials are generally known to possess higher surface area than SBA-15 materials, the later showed higher catalytic activity. Therefore, in this reaction the surface area wasn’t the determining factor. Instead, higher redox catalytic activity was observed when the niobium was in-situ impregnated. To summarize, the redox catalytic activity scale; MCM-41 <
(0.5 M) <
(1 M) < MCM-41/Nb (3%) < MCM-41/Nb (5%) < Nb- MCM-41, the same redox reactivity scale can be applied to all of SBA-15 catalysts.
![]()
Table 4. Catalytic evaluation of SBA-15 catalysts.
3.5. Catalytic Evaluation of Oxides (ZrO2, TiO2, HfO2)
The oxidation reaction of 1-octanol was also tested with the other catalysts that we previously prepared (ZrO2, TiO2, HfO2) [6] . These catalysts did not show oxidant properties and almost no esters were formed. The three catalysts (ZrO2, TiO2, HfO2) had the same catalytic activity, where when sulfated only dioctyl ether (7) was observed, and when impregnated with niobium it showed a very small conversion (<5%) of 1-octanol into products (8), (9) and (10). In general the conversion rates of 1-octanol (6) into dioctyl ether (7) were between 40% - 50% for all three catalysts (ZrO2, TiO2, HfO2), only when oxides were sulfated. On the other hand the redox catalytic activity of these catalysts gave negligible conversions (<5%). From these observations we concluded that this lower redox catalytic activity was probably caused by the much smaller surface area and the smaller pore sizes of (ZrO2, TiO2, HfO2) in comparison with MCM-41 and SBA-15 catalysts.
3.6. Proposed Reaction Pathways of 1-Octanol
In the oxidation reaction of 1-octanol, various interesting products were obtained; dioctyl ether (7), octyl octanoate (8), peroxyacetal (9) and octanal (10). We believe that the reaction took a radical mechanism, since peroxides were detected in the reaction medium using “Peroxyde 25” test sticks. With all reactions showing formation of the products (8), (9) and (10), the test was positive. When a certain reaction gave only dioctyl ether (7), the test was negative, indicating that ether formation done by the usual way under acidic conditions was provided by the surface Brönsted acid sites of our catalysts with no radicals formed.
The octanal (10) was identified by 1H NMR of the crude after reaction completion, from its characteristic triplet at 9.7 ppm.
In order to understand how the octyl octanoate (8) and peroxyacetal (9) were formed, several other experiments were conducted. First 1-octanol was reacted with octanal (10) 2:1 ratio and the mixture was catalyzed by
(0.5 M) that gave the most interesting results, (0.5 M) in Scheme 3, all under the same experimental conditions used in Section 3.1.
In this reaction high selectivity for octyl octanoate (8) was observed but with
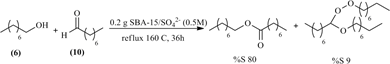
Scheme 3. Reaction between 1-octanol and octanal.
low 1-octanol (6) conversions and no dioctyl ether (7) formation. “Peroxyde 25” test was positive indicating the presence of peroxide intermediates.
Another experiment was conducted, where a pure sample of dioctyl ether (7) put in contact with the catalyst
(0.5 M) with no other reactants in Scheme 4 under the same previous reaction conditions.
In this reaction, low conversions were observed. Traces of the products formed octyl octanoate (8) and octanal (10), were a good indication that this is the reaction pathway taken by the dioctyl ether (7) in presence of the catalyst
(0.5 M).
Oxidation of 1-octanol (6) was done using a high catalyst loading, the idea was to see if relatively large scale reactions could improve 1-octanol (6) conversion and octyl octanoate (7) selectivity, but instead only dioctyl ether (7) was formed in Scheme 5.
This observation lead us to believe that the interface between three phases: the liquid (1-octanol), the solid (catalyst) and the gas (ambient air), played a key role in determining the reaction selectivity pathway.
So after having doubted on the influence of the surrounding air on the reaction, an experiment was realized under nitrogen gas (N2) to see if it will affect product selectivity in Scheme 6.
When the oxidation of 1-octanol (6) was performed under nitrogen, only dioctyl ether (7) was obtained. This is a clear indication that the oxygen (O2) in atmospheric air is an important factor in the reaction.
After all these experiments and the peroxide 25 tests, a suggested reaction mechanism was proposed in Scheme 7.
Oxidation of 1-Octanol under Pressure
After these observations and results, oxygen plays a key role in the reaction towards octyl octanoate (8) and peroxyacetal (9) formation. We also tried the reaction under air pressure in Scheme 8 using a pressure reactor (autoclave).
Since it was the
(0.5 M) catalyst that previously showed good conversion rate of 1-octanol into esters, we chose it for testing under air pressure in Table 5.
When the reaction was realized under air pressure, a small increase in 1-octanol conversion was observed, which improved selectivity for octyl octanoate (8) and decreased dioctyl ether (7) selectivity. This showed that this reaction could be improved (increasing octyl octanoate selectivity). We were skeptical about the results because after each reaction there was a huge part of 1-octanol lost by evaporation (reactor leaking), so the conversions and selectivity were over estimated.
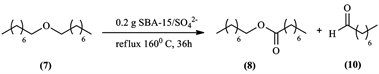
Scheme 4. Reaction of dioctyl ether in presence of SBA-15/SO42- (0.5 M).
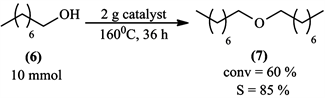
Scheme 5. Reaction of 1-octanol using large scale.
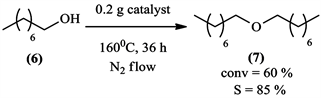
Scheme 6. Oxidation of 1-octanol under nitrogen.
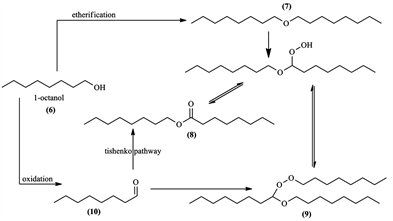
Scheme 7. Proposed reaction pathway and mechanism of 1-octanol transformations.
On another hand, we had concerns about the safety of conducting this reaction under oxygen pressure, since it requires very high temperatures and that there are unstable peroxides present in the reaction medium, so these obstacles prevented us from trying the reaction under pure oxygen pressure.
4. Conclusions
Porous oxides (ZrO2, TiO2, HfO2), have shown mild acidic properties when sulfated, evidenced by the conversion of 1-octanol into dioctyl ether, and very weak redox properties when impregnated with niobium, caused by the small surface area of these oxides.
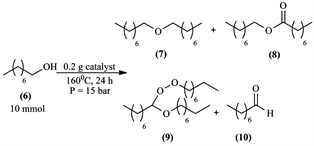
Scheme 8. 1-octanol oxidation under air pressure.
![]()
Table 5. Oxidation of 1-octanol under air pressure (P = 20 bar).
On the other hand, with the siliceous mesoporous catalysts that we prepared, different catalytic activities were compared to that obtained in the etherification reaction of 2-naphtol. In fact, both supports (MCM-41 and SBA-15) showed weak catalytic redox properties. This catalytic redox property was enhanced when the support is sulfated and specially when impregnated with niobium. The superior catalytic activity of mesoporous materials (MCM-41 and SBA-15) is attributed to the larger surface area which is important for better distribution of active species. Also all catalysts showed slightly better activity when in situ impregnated with either sulfate ions or niobium. For future work, more testing should be done under pressure and other transition metals could be impregnated on MCM-41 and SBA-15 supports. More advanced characterizations of the catalysts should be done, in order to establish a more clear correlation between structure of catalysts and active sites with catalytic behaviors. Nevertheless, we have demonstrated that oxidation of alcohol by air with redox acid catalyst may lead via a Tishenko reaction, to esters. This new reaction may be of interest specially if continuous flow reaction is performed in order to limit safety concern and may lead to higher conversion.
Acknowledgements
This work was funded by the Lebanese University (Lebanon) and Claude Bernard University (France) in the framework of the cooperative research program between these Universities. Preparation and characterization of the catalysts were done in EDST (Ecole Doctoral des Sciences et Technology-Liban) and catalytic tests were performed in CASYEN (CAtalysis, SYnthesis and Environment-France).