Optimization Study of the Removal of Atrazine from Aqueous Solution on to Composite Activated Carbon-Silver Using Response Surface Methodology ()
1. Introduction
Atrazine (2-chloro-4-ethylamine-6 isopropylamino-s-triazine) is a selective triazine herbicide frequently used in agricultural sector. Contamination of water and soil by atrazine has a negative impact on aquatic ecosystems and induce severe hormonal disturbances in amphibians [1] . Thus it is classified as a potential human carcinogenic by USEPA (United States Environmental Protection Agency) and the main source of human exposure is the consumption of contaminated groundwater [2] . Therefore, the European Union legislation allows a very low concentration of atrazine in drinking water (0.1 ppb) and USEPA has also set a maximum contaminant level at 3 ppb for it [3] . It becomes important to clean atrazine from groundwater.
One effective alternative to eliminate this recalcitrant compound could be the adsorption process. Adsorption is a process whereby a contaminant adheres to the surface of an adsorbent, such as activated carbon, due to hydrophobic and electrostatic interactions between the adsorbate and the adsorbent [4] . Activated carbon (AC) is a carbonaceous material that possesses a highly developed porosity that allows its use in wide range of applications. Some of the most important uses dealt with water treatment [5] .
Activated carbons are widely used to adsorb organic micropollutant from liquids or gas [6] . They can be obtained from various precursors such as oil palm shell, argan shell, coconut shell, peat, sugar canne bagasse. Sorption studies of atrazine have mainly been limited to activated carbon. But the presence of active silver metal onto impregnated activated carbon surface can greatly affect the adsorption affinity since inorganic compounds with the multiplication of the application and properties. Some other factors such as pH, temperature and ionic strength pH could have a significant effect in the adsorption of atrazine. For this reason, the Response Surface Methodology was used to optimize the impregnation of silver on the surface of the activated carbon for atrazine adsorption (response). The activated carbon thus obtained will be used for atrazine removal from liquid phase. Doehlert designs are easily applied to optimize variables and was selected to study firstly the effects of activated carbon preparation/silver variables (concentration of AgNO3, temperature and time of impregnation); secondly the effects of atrazine removal variable (pH, temperature and ionic strength) on the response. Thus; the objective of this work was to optimize the preparation of composite activated carbon/silver for the removal of atrazine.
2. Materials and Methods
2.1. Preparation of the Composite
The Oil palm shells were collected locality from Bafangin the West region of Cameroon. The precursor was cleaned several times with ionized water and sun dried. Then, they were crushed and sieved to particle sizes ranging from 2.0 - 2.5 mm. The dried residues were carbonized in a furnace tube (Carbolite1200C UK) at 400˚C for 2 h under a flow of N2 gas, at a heating rate of 10˚C/min. Before, it was activated at 850˚C for 6 h (heating rate of 10˚C/min) under steam (0.1 mL/min) and cooled to room temperature. After activation, the samples were washed in distilled water, dried, ground, and sift to obtain powder with particle size less than 50 µm. To functionalize the surface of activated carbon, the activated carbons were treated with HNO3 (1 mol/L).
Activated carbon-silver composites were prepared from the suspension of 0.6g of activated carbon in 1.6 mL of water at desired concentration (0.05 - 0.1 mol/L) of silver (AgNO3). The mixture was introduced in the furnace tube (at hydrothermal carbonization) in the dark. After 1 h, the temperature was increased in the range (188˚C - 292˚C) at the desire time in the domain of (1.3 - 3.7 h). Then, the composite was washed with distilled water and dried at 105˚C until constant weight and kept in a hermetic bottle for further test.
2.2. Characterization of the Composite
The composites were characterized using powder EDX for the chemical composition of the AC-AgFT-IR spectroscopy was applied in order to identify the functional groups and chemical bonding on the adsorbents. For this purpose, spectra were determined between 4000 and 400 cm−1 using an FT-IR spectroscope (Spectrum Vertex 70 DTGS). The morphological analysis of the activated carbon was performed by Scanning Electron Microscopy (SEM) (JEOL JSM-5400, Japan).
2.3. Experimental Design
Response Surface Methodology (RSM) is a collection of mathematical and statistical techniques that are useful for modeling and analysis of problems in which a response of interest is influenced by several variables [7] . In this work, The Doehlert experimental design was applied in order to reduce the number of experiments to study the variables of impregnation of activated carbon by silver in one hand and the variables for adsorption of atrazine from aqueous solution using the composite activated carbon-silver (AC-Ag) in a batch process in another hand. Doehlert matrices present the advantage of being easily expanded in both the variables space and the experimental space. This method was chosen for fitting a quadratic surface with a minimum number of experiments. It helps also to analyse the interaction between the effective process parameters and to identify the factor settings that optimize the response [8] .
In the present study, the composite AC-Ag was prepared using hydrothermal carbonization by varying the preparation variables using Doehlert experimental design. The variables studies were: concentration of AgNO3(X1); impregnation temperature (X2) and impregnation time (X3). For the adsorption of atrazine, three independent tests were chosen for the statistical experimental design as follows: temperature (˚C) (X1), pH (X2) and ionic strength (atrazine/CaCl2) (X3). The range and levels of the factors which were varied according to the experimental design are given in Table 1 and Table 2.
![]()
Table 1. Experimental design Matrix, operating conditions and the corresponding experimental response for impregnation activated carbon (AC-Ag).
Y1: Experimental response; Exp: experiment.
![]()
Table 2. Estimated values of coefficients for Y1.
The experimental design matrix of 17 experiments and the results are given in Table 3. Each row represents an experimental run, and each column represents the variables tests. The response analyzed was the atrazine uptake. The responses are assuming to be affected by three variables and the experimental data were analyzed to fit the following second order polynomial equation:
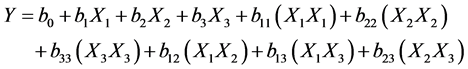 (1)
(1)
where, Y is the predicted response, b0 a constant coefficient, bi a linear coefficient, bii a quadratic coefficient, bij an interaction coefficient, X1 coded variables of concentration of AgNO3 and adsorption temperature. X2 coded variables of Impregnation temperature and pH and X3 coded variables of impregnation time andratio atrazine/CaCl2) of the impregnated activated carbon variables and adsorption of atrazine variables respectively. The experimental data were analysed using software named NEMROD (New Efficient Methodology of research using optimal design); for regression analysis, to fit the equations developed and also to evaluate the statistical significance of the equations obtains [9] .
2.4. Adsorption Experiment
The batch experiments for the adsorption studies were carried out at room temperature in conical flask of 150 mL. For each run, 10 mg of the adsorbent was introduced into the flask containing 100 mL of the atrazine solution at initial concentrations of 20 mg/L. The shaker was set at a desired temperature (15˚C - 35˚C) at a speed of 250 rpm and the solution at a desired pH (3.4 - 8.6) and at a desired ratio atrazine/CaCl2 (0.37 - 1.83). After reaching the equilibrium, AC-Ag were separated from the aqueous solution using filtration method with 0.45 µm what man cellulose nitrate menbrane. Then, the analysis of the residual solution was performed by UV-visible spectrophotometer Secomam at 225 nm. The quantities adsorbed at equilibrium; Qe (mg∙g−1) were calculated according to:
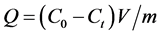 (2)
(2)
where, C0 and Ce (mg/L) are the initial and equilibrium concentrations of atrazine in solution, respectively, V (L) is the total volume of the solution, and m (g) is the adsorbent mass.
3. Results and Discussion
3.1. Development of Regression Models Equation
The examination of the given results in Table 1 showed that, the adsorption capacities of atrazine (Y1) ranged from 270 to 384.62 mg/g. The adsorption capacity of atrazine has a high value for [AgNO3] = 0.063 M; at 223˚C and at 1.3 h (experiment 8), whereas, the lowest value was obtained at the same [AgNO3] = 0.063 M but now at 257˚C and 3.7 h (experiment 11).
Nevertheless, for the removal of atrazine in function of medium conditions, Table 3 showed the capacity of the carbon samples to adsorb atrazine which varies between 157.69 and 209.45 mg/g.
3.2. The Effect of Factors on the Atrazine Adsorption Capacities (Y1)
The polynomial model equation in terms of coded factors is given as:
![]()
Table 3. Experimental design Matrix: operating conditions and the corresponding experimental response for the adsorption of atrazine in function of medium conditions.
 (3)
(3)
The quality of the developed model was evaluated based on the correlation coefficient, R2 and the adjusted R2 indicating that the variability in the response could be explained by the mathematical model [10] . In this case, the correlation between the theoretical and experimental responses, calculated by the model is satisfactory: R2 = 0.984 and adjusted R2 = 0.964. In the case of response Y1, the positive sign in front of the terms indicates synergistic effect whereas negative sign indicates antagonistic effect [11] [12] . Table 2 shows the analysis of variance (ANOVA) of response Y1, it is clear that, the impregnation time (b3 = −42.360) and carbonization temperature (b2 = −28.168) have a significant effect for the adsorption of atrazine. But the concentration of AgNO3 has no significant effect on this response. The quadratic term of concentration of silver (b11 = 53.533), impregnation time (b33 = 31.497) have a significant effect on the response and the interaction between impregnation temperature and time (b23 = −67.073) imposing the most effect.
Figure 1(a) and Figure 1(b) shows the two and three dimensional response surface which were constructed to present the most important factors on the atrazine removal by AC-Ag
The Figures shows that, atrazine adsorption increases when the [AgNO3] in-
![]()
Figure 1. (a) Variation of the Atrazine adsorption (Y1 mg/g) in the plan Time (h)-[AgNO3] (mol/L); (b) Variation of the Atrazine adsorption (Y1) in the plan Temperature (˚C)-Time (h).
crease. It is found that, atrazine exists almost exclusively as neutral molecules, and the weak forces such as van der waals forces, hydrogen bonds and hydrophobic interaction would involve in the reciprocity of atrazine with activated carbon [3] . Similar results have been reported by Emily et al. [13] ; Park et al. [14] ; Quing-Hui and Hong-Xiao [15] .
From Figure 1(b), at low temperature (T = 180˚C), Y1 increase when time increases from 1 h to 4 h in contrast at high temperature (300˚C), Y1 decrease when time increases. However, at low temperature, the iron was not crystallized in the activated carbon surface, which gave free active sites on the adsorbent that were favorable for the adsorption of atrazine. The second observation indicates that, the uptake of atrazine on activated carbon was an exothermic process [16] . Nevertheless, Urena-Amate et al. [17] explained this phenomenon by the fact that at high temperature, the formation of the unions between the activated carbon surface and the atrazine molecules will be diminished. Probably due as the content of iron in the composite increases because the surface area decrease caused by the presence of a high quantity of iron oxide in the activated carbon/Iron oxide occupying the active sites avoiding the contact of atrazine molecules to adsorption sites on the carbon surface [2] .
3.3. Effect of Medium Condition on Removal of Atrazine (Y2)
This response is described by the following equation:
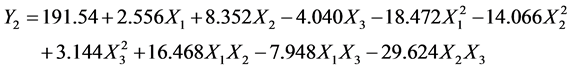 (4)
(4)
With a significant correlation coefficient (R2 = 0.929 and R2 adjusted = 0.893). The coefficients estimated from the results are displayed in Table 4. The analysis of the different effects showed a significant effect of pH for the removal of atrazine. The interaction b13 and b23 have a significant effect on the capacity of atrazine adsorption (Y1).
This Figures 2-4 showed the variation of atrazine adsorption as a function of different factors. Figure 2 shows the combined effect of pH and temperature on adsorption of atrazine. It is evident from the Figure 2 that the adsorption capacity of atrazine increases when the temperature and pH increases. This result agrees with recent studies which reported that removal efficiency of micropollutants is generally lower at low temperature [4] . But this response decreases when rAyraz/CaCl2 and temperature increases (Figure 3), it may be due, to an increase in the atrazine solubility in this step, implying a decrease of hydrophobics interactions [18] . The net effect of inorganic salts on atrazine sorption can be explain by two opposite factors: as increase in the solution, ionic strength can partially dissociate hydroxyls groups on adsorbent, disfavoring the H-bond with atrazine. Moreover, the addition of inorganic salts reduces the double layers thickness and strengthens the hydrophobic interactions that facilitate atrazine sorption [19] . Therefore, the increase in temperature caused a decrease in ad-
![]()
Table 4. Estimated values of coefficients for Y2.
![]()
Figure 2. Variation of atrazine adsorption capacity (Y2; mg/g), as a function of pH (X2) and Temperature (X1).
![]()
Figure 3. Variation of atrazine adsorption capacity (Y2; mg/g), as a function of r Atraz/CaCl2(X3) and Temperature(X1).
sorbed atrazine, indicating that, the process was exothermic [17] . Nevertheless, Figure 4 shows the interaction between pH and r (Atrz/CaCl2). Indeed, at low pH the increase of r(atraz/CaCl2) increase Y2. In contrast at high pH, the increase of r (atraz/CaCl2) decreases Y2. The observed ionic strength effects on atrazine adsorption reflect a cooperative effect both, Ca2+ and Cl−. At low pH, positively charged surface would favor ion pair formation between Cl−and AC/Ag. But, atrazine maintains its non-ionized form and is adsorbed onto AC/Ag. The increase of Y2 when of r(atraz/CaCl2) increases at low pH is probably due to the best affinity of atrazine onto AC/Ag surface and we can conclude that Cl− has a weak binding with AC/Ag. For this reason, Jianghua et al., said that, acidic condition is more favorable to this adsorption process [20] . However,
![]()
Figure 4. Variation adsorption capacity of atrazine (Y2; mg/g), as a function of pH and rAtraz/CaCl2.
at high pH, atrazine can be converted to a negative charge from the protonated base in basic solution. The number of negatively charged adsorbent sites increased at pH > 6.93, limiting the adsorption of atrazine. But the increase ofr (atraz/CaCl2) at high pH enhances ion pair formation between Ca2+ and AC/Ag who is charge negatively. Consequently, electric repulsion between the negatively charged AC/Ag surface and atrazine might have occurred at high pH [21] .
3.4. Process Optimization
The preparation of composite AC/Ag was done under the experimental conditions given in Table 1 and the experimental conditions of removal of atrazine as a function of medium condition given in Table 3. The optimum condition for activated carbon composite were obtained using preparation conditions of: 0.063 mol/L concentration of silver, 223˚C impregnation temperature and 1.3-hour impregnation time which conducted to Y1 equal at 384 mg/g. Whereas, the optimum adsorption of atrazine under effect of medium conditions were obtained at: 25˚C, pH 7.7 and 0.37 rAtraz/CaCl2 for Y2 of 209 mg/g. It is clear that the medium conditions have a high effect on the efficiency of atrazine at 20 mg/L of concentration. From the literature, Llado et al, used a commercial activated carbon Filtrasorb 400 for the adsorption of atrazine and found that the maximum adsorption capacity was 212 mg/g at 40 mg/L concentration of atrazine [6] . Thus, the composite AC/Ag prepared in this work is suitable to be used for the removal of atrazine in aqueous solution.
3.5. Characterization of Activated Carbon Prepared under Optimum Condition
Figure 5 shows the EDX spectrum of the defined area of AC-Ag. The characteristic peaks of Ag and Carbon were clearly observed, indicating the existence of
![]()
![]() (a) (b)
(a) (b)
Figure 6. (a) The SEM image of AC; (b) The SEM image of AC-Ag.
Ag at 44.3% and Carbon at 55.7%. The SEM images of AC and AC-Ag are shown in Figure 6. We can observe many orderly and developed pores due to the effect of the steam activation produced on the oil palm shell in a first time demonstrated by homogenous circle shapes with same sizes of uniformly distributed pores (Figure 6(a)). But, after impregnation with silver, we can observe that, Ag particles were covered on the surface of AC hindering some of the pores (Figure 6(b)).
The Fourier Transform Infrared (FTIR) spectra of AC and AC-Ag (Figure 7) indicates the formation of large number of hydroxyl or phenolic hydroxyl groups on the surface of AC at 3411 and 3451 cm−1 [20] . The O?H stretching vibration (3408 - 3452 cm−1) and C?OH stretching vibration (999 - 1027 cm−1) were more obvious and broader in all samples. The three peaks located at about 1654 and 1575 cm−1 could be assigned to C=O vibration and in-plane C=C stretching vibration of aromatic ring, respectively for AC-Ag and AC, which supported the concept of aromatization of the activated carbon. The bands in the range 1000 - 1500 cm−1, which include the C-OH stretching and OH bending vibrations, imply the existence of large numbers of residual hydroxy groups (OH) and carboxylate groups (COOH) [22] . The OH and COOH can react with metal ions to form metal nanoparticles [23] . It can be concluded that the surface functional groups changed largely. Moreover, aromatic C=C stretching (skeletal ring vibration) at about 1654 cm−1 increased sharply.
Figure 8, show that the pHPzc = 6.93; In this case, the surface charge of AC/Ag
was positive when pH is below pHPzc and negative when pH is above pHPzc.
4. Conclusion
The composite AC-Ag has been prepared for adsorption of atrazine. The impregnation condition of AC-Ag and the removal of atrazine have been optimized using Response Surface Methodology (Doehlert design). The second polynomial equation has been found to fit most satisfactorily the model predicted according to the correlation coefficients obtained. The obtained adsorption capacities were 384 mg/g for optimum condition of impregnation and 209 mg/g for a medium condition for adsorption. It is clear that the medium condition has the less effect on the adsorption of atrazine. Finally, it can be concluded that AC-Ag has an excellent potential of adsorption of atrazine. Therefore, it can be efficiently used for treatment of industrial effluent containing these pollutants.
Acknowledgements
Dr. BEAS of the University of Technology of Johannesburg is highly acknowledged for his assistance in analysis and interpretation, remarks and suggestions in the write up of this paper. The authors thank the Applied Organic Chemistry Laboratory of the Chemistry Department, Faculty of Science Semlalia, Cadi Ayyad University of Morocco, for the materials and logistics support. And finally thank all the members of the Research Unit “Adsorption and Surface” of the Applied Physical and Analytical Chemistry Laboratory of the University of Yaoundé I.