Hydro-Adsorption Study by Dynamic Laser Speckle of Natural Zeolite for Adsorbent and Fertilizer Applications ()
1. Introduction
The minerals based on aluminosilicates such as clays (kaolinite, montmorillonite) and zeolites (clinoptilolite) are abundant and inexpensive [1] . Zeolites present a lot of peculiar characteristics, one of them is the property of adsorbents for removing bacteria and pollutants [2] [3] [4] [5] . Clinoptilolite is a natural, non-toxic zeolite with strong adsorptive and ion-exchange capacity [5] [6] . Its structure is formed with a 3D framework, built up by tetrahedral TO4 (T = Al3+, Si+4), sharing all their vertexes linking together and forming different rings of 6, 8, 10 and 12 member [5] [6] . The parameter framework density, FD (number of the T atoms per 1000 Å3 of volume) is useful for distinguish Zeolites from other dense tecto-silicates, ranging between 12 and 20 for Zeolites [2] . The treatment of the zeolite surface with concentrated acids or bases also modifies their hygroscopic properties [5] [6] . It is well known that the presence of hydrogen bonding interactions, and electrostatic forces of attraction on the surface of zeolites modified with anionic and/or cationic species has a significant effect on the process of microorganism elimination [3] [4] . Also, the pronounced selectivity of clinoptilolite for large cations, such as ammonium and potassium, has also been used in the preparation of chemical fertilizers that improve the nutrient-retention ability of the soils [7] . In our previous studies, this geomaterial produced an optimal degree of exchange with ammonium NH4+ (~90% of the theoretical CEC) and showed a good performance as a slow nitrogen release fertilizer applied in crops grown with two types of Argentine soil. These results revealed the potential of ammonium clinoptilolite system in sustainable agricultural production [8] .
Natural and modified zeolites and their applications have been widely studied, so determination of textural and structural properties has been of great interest. Usually, the hygroscopic properties of the zeolites can be characterized by the well-known TG-DTA, XRD, FTIR and BET methods [3] [4] [5] . Recently dynamic laser speckle technique (DLS) has been used for estimate the hygroscopic capacity in commercial silica (SiO2) and clays with different textural properties. The DLS represents a technologically advanced and methodologically accurate method based on the optical random interferometric phenomenon named “speckle”, produced when a laser light illuminates a rough surface [9] [10] . This effect shows the temporal evolution of materials speckle patterns during water adsorption and a correlation of the speckle activity with the textural properties. This analysis can be considered as an alternative method to study different porous materials of interest such as absorbents or catalysts supports [11] [12] .
The present work addresses the implementation of dynamic laser speckle technique to the hydro-adsorption analysis of natural zeolite, clinoptilolite, and their acidic, basic and thermally treated forms. This technique can be very useful to estimate its hygroscopic capacity.
In this paper, experimental DLS data were fitted using the Hawkes and Flink [13] ; Azuara et al. [14] [15] [16] and Peleg [17] - [23] theoretical adsorption methods. These fitting were improved modifying the Peleg’s equation.
In view of the versatile importance of the DLS technique, we here report its application together with physicochemical analysis such as XRD, FTIR and BET method.
2. Materials and Methods
The natural granular clinoptilolite zeolite used in this study was obtained from La Rioja (northwestern province of Argentine). A sample with the chemical composition given in Table 1 was selected. Data were obtained by Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES) by LiBO2/Li2B4O7 fusion (ACME Company, Canada) [24] [25] .
The natural zeolite was ground manually in order to obtain a granular form as a solid powder, then 1 g of the solid powder was treated with 10 mL of ammonium hydroxide (1 M) solution for basic modification and 10 mL of concentrated nitric acid (1 M) for the acid one. Both samples were stirred during 12 hours and heated at 70˚C.
Solid samples were separated from the solution by filtration and washed with distilled water several times till the effluent became neutral to pH paper.
On the other hand, two samples of 1g of original zeolite were thermally treated at 250˚C and 500˚C for 2 h in air. The zeolites (Z, in what follows) natural and the zeolites modified: Z-H+ (acid form), Z-NH4+ (basic form); Z-250 and Z-500 (zeolites heated at different temperatures). Characterization of original and treated zeolites was performed by Electronic microscopy using a Philips SEM 505 combined with semiquantitative analysis by energy dispersive X-ray analysis (EDS) by an analyzer EDAX 9100. Diagrams of X-ray diffraction powders (XRD) were registered using a Philips PW 1714 with a CuKα radiation and Ni filter from 2θ = 5˚ to 60˚. Infrared FTIR Spectra were obtained by a Equinox 50 FTIR equipment, from 4000 to 400 cm−1 wave-numbers. Surface areas and porosity of samples were determined by physical N2 adsorption at 77 K (BET method [26] ) using a Micromeritics apparatus ASAP 2010.
2.1. Dynamic Laser Speckle (DLS) Technique
A typical granular interference pattern named “speckle” is observed [9] [10] when a coherent beam coming from a laser illuminates a rough object. Besides, a laser light scattered from diffuse objects produces a similar pattern.
If this surface presents some type of local movement, then the intensity pattern evolves in time. This phenomenon, known as “dynamic laser speckle” (DLS), can be observed in biological samples [9] and in non-biological industrial processes, including the drying of paint, corrosion and heat exchange [10] . This effect takes place when the sample changes its surface properties due to the movement of the scattering centers; changes in the optical path due to variations of refractive index; configuration changes or combination of these situations [10] . The temporary evolution of the speckle patterns is correlated with the “activity” of the sample and may provide an interesting tool to characterize the parameters involved in these processes. In this case, the speckle patterns showed a high activity for the initial hydro-adsorption process and it is minimal when this process is complete.
![]()
Table 1. Chemical analysis of bare clinoptilolite by ICP-AES analysis. (Majority elements (as % oxides)).
LOI: Loss on ignition.
We propose a DLS method to characterize the water adsorption of the zeolite. We employed a 10 mW He-Ne laser to illuminate the samples. Dynamic speckle pattern were recorded with a CCD camera connected to a frame grabber to digitize the images as shown in Figure 1. During this process, 30 mg of each zeolite sample were impregnate with 10μl distilled water. The total experiment was carried out at 19˚C and 60% of humidity.
We used Oulamara et al. method [9] to show the temporal evolution of speckle. In our case, 320 successive images (25 frames per second) of dynamic speckle were recorded for each state of the adsorption process and selecting a column of them. With these columns, a pseudo-image of 320 × 240 was constructed and so-called Temporary History Speckle Patterns (THSP).
The speckle activity of the sample changes their intensity in the horizontal direction. Therefore, when a phenomenon shows low activity, time variations of the speckle pattern are slow and the THSP shows elongated shape. When the phenomenon is very active, the THSP resembles an ordinary speckle pattern. See Figure 2.
The moment of inertia of the co-occurrence matrix method [10] was used for a quantitative estimate of THSP. The moment of inertia is defined by:
 (1)
(1)
where 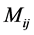 represents the number of occurrences of the gray-level i followed in the time direction by gray-level
represents the number of occurrences of the gray-level i followed in the time direction by gray-level 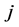 in the THSP image, which forms an intermediate matrix called a co-occurrence matrix.
in the THSP image, which forms an intermediate matrix called a co-occurrence matrix.
In our case, the initial value of I decreases as the water is adsorbed by the zeolite surface until reaching the steady state, thus it is directly related to the adsorbed water amount.
2.2. Adsorption Theoretical Models
For modeling the amount of adsorbed water on solids vs. time, different models have been reported [13] - [23] .
![]()
Figure 1. Experimental set-up for dynamic speckle technique.
![]()
![]() (a) (b)
(a) (b)
Figure 2. Temporal History of Speckle Pattern (THSP), (a) high activity (initial hydro-adsorption process), (b) low activity (process is complete).
Hawkes and Flink [13] studied the kinetics of water loss and solute uptake for solutions of differing composition and concentration. Several of the osmotically pre-concentrated freeze dried apple slices for organoleptic acceptability were evaluated using the following equation:
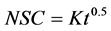 (2)
(2)
where 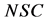 is the normalized solids,
is the normalized solids, 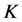 is defined as the mass transfer coefficient and
is defined as the mass transfer coefficient and 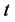 is the time.
is the time.
Azuara et al. [14] [15] [16] developed a two-parameter equation from mass balance considerations. This equation was used to predict the kinetics of osmotic dehydration and the final equilibrium point in cereals and fruits [14] :
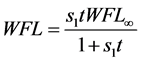 (3a)
(3a)
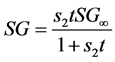 (3b)
(3b)
In Equation (3a), WFL is the water lost by the foodstuff at time t,  is a constant related to water loss and
is a constant related to water loss and 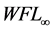 is the water lost at equilibrium. In Equation (3b),
is the water lost at equilibrium. In Equation (3b),  is solids gained by the foodstuff at time
is solids gained by the foodstuff at time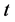 ,
, 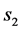 is a constant related to solids gain and
is a constant related to solids gain and ![]() is the amount of solids gained at equilibrium.
is the amount of solids gained at equilibrium.
Finally, Peleg [17] developed an empirical model for sorption curves in the form of moisture vs time relationships of milk powder and rice, exposed to moist atmosphere or soaked in water. The model enabled prediction of moisture contents after long exposure from experimental data obtained in a relatively short time, i.e., before the moisture level appeared to reach a plateau. The model implied that the moisture equilibrium was somewhat higher than that determined on the assumption that the sample reached a constant weight, [17] - [23] :
![]() (4)
(4)
where ![]() is the moisture after time
is the moisture after time![]() ,
, ![]() the initial moisture, and
the initial moisture, and ![]() are constants.
are constants.
In this paper, we try to use the expressions of the reported models applied to the data (moment of inertia) obtained by dynamic speckle technique.
Taking into account the above considerations, Equations (2)-(4) must be modified and turned into:
“![]() ” Hawkes and Flink variable is set as the remaining water instead of adsorbed water, then Equation (2) could be stated as:
” Hawkes and Flink variable is set as the remaining water instead of adsorbed water, then Equation (2) could be stated as:
![]() (5)
(5)
where “![]() ”, represents the amount of initial water standardized.
”, represents the amount of initial water standardized.
So, in dynamic speckle technique we use the expression:
![]() (6)
(6)
where ![]() is the moment of inertia as a function of time,
is the moment of inertia as a function of time, ![]() is the initial moment of inertia and
is the initial moment of inertia and ![]() represents the water remaining on the surface of the zeolite,
represents the water remaining on the surface of the zeolite,
Azuara’s et al. model equation became:
![]() (7)
(7)
where ![]() is the steady state moment of inertia.
is the steady state moment of inertia.
Peleg’s model equation became:
![]() (8)
(8)
where ![]() is the steady state moment of inertia and
is the steady state moment of inertia and ![]() is the initial variation of moment of inertia.
is the initial variation of moment of inertia.
3. Results and Discussion
3.1. Physicochemical Characterization
Clinoptilolite used in this work is a heulandite-type, with chemical composition (Na,K)6(Al6Si30O72)∙20H2O previously reported [8] [24] [25] . Data are included in Table 1.
The framework topology of this mineral consists of a two dimensional pore system formed by 8-ring channels linking together [2] . This channels disposition allowed to zeolite has good adsorption properties for CO2, SO2, NH3 and NOx gases [27] [28] [29] [30] .
The cation exchange capacity (CEC) of this material has been previously determined and a value of 330 meq/100 g was found [28] .
Chemical and physical properties of the zeolite can be modified with either inorganic basic or acid solutions. This fact mainly depends on the Si/Al ratio.
The acid treatment conduces to the alumination process from the alumino-silicate structure removing Al3+-ions progressively. On the other hand, an increase of the temperature or alkalinity reduces the silica content, due to dehydration processes and SiO2 dissolution. This treatment also promotes the process of ion exchange cation (Na+ or K+)-H+. In basic treatment using, amoniacal solution, the silanol groups surface (hydroxyl) SiO2∙OH, acquires a great concentration of -OH groups. Therefore, under this condition, the NH4+ cations can be easily exchanged by other ions like Na+ or K+. All these reactions produce chemical surface modifications that are evidenced by changes in the composition, in textural and structural properties, which could be analyzed by different physical-chemical analysis techniques.
Textural properties, surface area, volume and pores size, have been analyzed with nitrogen physic-sorption isotherms by BET model. The measure was carried out for pure and modified samples with acid, basic treatment included the two calcinated samples. The results found together with some SEM-EDS chemical data are reported in Table 2.
The values obtained are typical for natural zeolites, however a noticeable change for zeolite calcinated at 500˚C was observed. In this case the SBET value was almost double and the pore size was lower than those the other samples.
Table 2 shows the composition of the minerals. It should be noted that the composition of the exchangeable cations, especially Na+ for Z-H+ and Z-NH4+ is lower than that of the unmodified mineral.
Figure 3 shows the comparative X-Ray diffraction patterns. The Z-N pattern is in agreement with the PDF 79-1461 previously reported [8] . All samples show the similar patterns, indicating that the structure does not change with the different treatments. However, X-ray diffraction lines of the calcinated samples are slightly shifted to lower 2θ probably due to the increase of the cell volume. On the other hand, the X-ray diffraction lines of the Z-H+ and Z-NH4+ samples are slightly shifted to the higher 2θ suggesting a decrease of the volume cell in agreement with the low percentage of the Na+ (see Table 2).
Figure 4 shows the infrared spectra of the samples. The bands observed around 3600 cm−1 and 1600 cm−1 are assigned to stretching and bending modes of the water molecules. The bands located at 3424 cm−1 are associated with the stretching of -OH groups from water molecules coordinated to cationic species, which are interacting with the covalent network that leads to hydrogen bonds of different sizes. The bands between 1200 and 400 cm-1 are assigned to the stretching and bending modes of the T-O units (T = Si or Al) due to the [29] TO4 tetrahedral groups (SiO4 and AlO4). The bands observed at 1206 and 607 cm−1 are assigned to the asymmetric and symmetric stretching
![]()
Table 2. Textural parameters by BET model and composition of Na, K and Si/Al by SEM-EDS for original clinoptilolite, modified and thermally treated forms.
![]()
Figure 3. Comparative XRD patterns for pure Z-N and treated samples Z-H+; Z-NH4+; Z-250 and Z-500.
![]()
Figure 4. FTIR spectra of pure and modified samples of zeolite (range: 4000 to 400 cm−1).
modes of internal tetrahedral. The bands at 1053 and 794 cm−1 are associated with the asymmetric and symmetric stretching modes of external linkages [30] , respectively.
The spectra of the acid form of the zeolite show that the external asymmetric mode νa T-O-T is lightly shifted to higher wave numbers respect to the natural zeolite. In this spectrum two new bands appear. One of them, located at 1384 cm−1 is attributed to asymmetric stretching modes of the NO3 bonds from the surface nitrate group which was generated by the HNO3 treating. The other one, observed at 955 cm−1 is assigned to Si-OH stretching mode of typical Brønsted acid site.
The spectrum of Z-NH4+ shows in 3900 to 3200 cm−1 range, bands assigned to the N-H stretching due to the presence of NH4+ bonded to the surface. The band at 1632 cm-1 results from the superposition of the ammonium component and the mode corresponding to the bending vibrations of adsorbed H2O. The presence of ammonium FTIR bands suggest the adduct formation between the ion and Brønsted acid sites of the zeolite [31] [32] .
FTIR spectra of the samples do not show significant changes with the temperature in agreement with the X-ray diagrams. This fact is probably due to the high thermal stability of the structure [33] [34] .
Table 3 shows the relevant bands and their tentatively assignment of the samples.
3.2. DLS Technique Applied to the Hydro-Adsorption Analysis of Pure and Modified Zeolites
Figure 5 shows the experimental data for original zeolite (Z-N) obtained with DLS technique (vs. time) (gray dots). Initial speckle activity decays monotonically to achieve stability in about 100 seconds.
Then, the experimental data were fitted using moment of inertia in Equations 6-8, and revealed the following values: a) Hawkes and Flink model [13] : [k = −12.9375, ![]() ,
, ![]() [Equation (6)], (short blue dashes), b) Azuara et al. model [14] [
[Equation (6)], (short blue dashes), b) Azuara et al. model [14] [![]() ,
, ![]() ,
, ![]() ,
,![]() ) [Equation (7)] and c) Peleg [17] [
) [Equation (7)] and c) Peleg [17] [![]() ,
, ![]() ,
, ![]() ,
,![]() ) [Equation (8)] were applied (long red dashes).
) [Equation (8)] were applied (long red dashes).
From Figure 5, it can be observed that the best fit is obtained with the Peleg’s and Azuara’s et al. models which fit the data with the same curve.
![]()
Table 3. Tentatively assignment of FTIR bands (ν cm−1) for pure and modified samples of zeolite.
Ref.: (a) External modes; (b) Internal modes.
![]()
Figure 5. DLS activity experimental values for natural zeolite (gray dots); Hawkes and Flink, [Equation (6)] in short blue dashes and Peleg and Azuara et al., [Equation (7), Equation (8)], in long red dashes; proposed second order approach [Equation (9)] in black line.
These mathematical approaches are equivalent and produced the same results with the experimental trend but with a high error data. This effect is probably due to the zeolite containing micro and small mesopores, which produce greater water adsorption rate, so the first-order equation did not fit the experimental results. Then, we propose a new approach modifying the equation of Peleg, extending to the second order:
![]() (9)
(9)
where![]() ,
, ![]() ,
, ![]()
![]() , and
, and![]() .
.
Figure 5 shows that the fitting of the data is substantially improved with this approach. Also ![]() coefficient is close to one, showing that our approach is the best fit for the experimental data.
coefficient is close to one, showing that our approach is the best fit for the experimental data.
Taking into account the good fit for Z-N, the method was also applied for Z-H+, Z-NH4+, Z-250 and Z-500 samples, in order to obtain the DLS activity (Figures 6 (a)-(d)). Data fitted to the experimental values are shown in Table 4.
Derivation of the Equation (9), the I rate of change vs time was obtained. Figure 7 shows the curves for all samples.
The I rate of change for the Z-N is the highest comparing with the other samples. (see Figure 7 black line). It reaches a faster saturation adsorption at small times.
However, the behavior is different for the other samples, especially for those thermally treated where the rate of change at small times is slow and saturation occurs at larger times for high temperature (Z-500). This effect is probably due to the decrease in the average pore size (see Table 2) because external (adsorbed water) and internal (coordinated water) dehydration were produced by the calcination processes.
Therefore, the reversible process will require the formation of new hydrogen-bridges to achieve rehydration of the pores. This process will be slower than for unheated samples. In previous report [34] , thermo-gravimetric analysis (TG) of clinoptilolites showed significant mass loss up to 500˚C, due to the loss of water coordinated to the intra-network ions (Na+ and K+). The complementary differential analysis (DT)
![]()
Figure 6. DLS activity for Z-H+; Z-NH4+; Z-250 and Z-500. Experimental values in gray dots and Equation (9) in dot line.
![]()
Table 4. Setting parameters for the moment of inertia (Equation (9)) for pure and modified zeolite.
![]()
Figure 7. ![]() rate of change vs. time for Z-H+; Z-NH4+; Z-250 and Z-500.
rate of change vs. time for Z-H+; Z-NH4+; Z-250 and Z-500.
revealed two endothermic peaks at 167 and 500˚C [35] . This effect is in agreement with the existence of two different kinds of water molecules, adsorbed and coordinated molecules, respectively. For that reason it is expected that the rehydration process for the sample heated at 250˚C will be faster than that at 500˚C.
Regarding acid and base modified samples, the I rate of change behavior for Z-H+ resulted similar to Z-N. However, this value is slightly higher for Z-NH4+ (Figure 7). This fact could be explained by the different sodium and potassium percentage (See Table 2), which resulted higher in the Z-H+ sample than in the Z-NH4+ one. Na+ and K+ cations are the normal sites for the intra-network water adsorption and acidic zeolite resulted from the exchange of these cations by protonated ion species (H+), giving rise to the formation of new hydrogen-bridges. This effect facilitates rehydration of Z-H+ intra-network pores and a good hydro-adsorption capacity.
For Z-NH4+, ammonium is also easily exchanged with Na+ and K+ ions, replacing the intra-network cations by “NH4OH” groups. This fact produces a steric effect in the pores and a slower rate of water molecules adsorption than the acid sites provided by Z-H+. This behavior is consistent with an intermediate speckle activity for Z-NH4+ (See Figure 7).
4. Conclusions
The dynamic speckle technique was employed in the study of natural zeolites and their thermally and chemically modified forms. This technique has been used for the first time for Zeolites material and resulted a very useful tool to compare the hydro-adsorption properties in different treatment Zeolites sample.
According the water adsorption process of the samples, the experimental results show the temporary evolution of the speckle patterns. The parameters of the optical configuration used to produce speckle have been fitted using an improved Peleg’s equation.
The good agreement between the experimental DLS results and calculated values can be considered as a potential low cost, non-destructive and simple method to study different materials of interest such as adsorbents or catalysts supports.
Acknowledgements
We are grateful to Lic. Mariela Theiler, Mrs. Graciela Valle and Eng. Edgardo Soto for their technical assistance.
The authors would like to thank the following institutions for funding this work:
CONICET (PIP 0003, 0771); CICPBA (Project 832/14) and Universidad Nacional de La Plata (Projects X606 and I172).