Site Selectivity of One Hydroxyl Group Bonded on the Surface of Finite (5, 0) Zigzag Carbon Nanotube ()
1. Introduction
Carbon nanotubes as well as other tubular nano materials have attracted considerable attention in recent years, among several low-dimensional physical systems [1] [2] . CNTs have significant potential for application incatalysis activity [3] [4] , molecular electronics [5] [6] [7] [8] , optics [9] and sensors [10] . However, in many applications, especially involving catalysis, adsorbents and sensors, for improved device performance and its commercial success we have to control structural integrity through chemical modification including the surface oxidation and surface functionalization [11] [12] [13] . This can be done by interaction of oxygen-containing groups such as hydroxyl (-OH), carbonyl (-C=O) and carboxyl (-COOH) with the surface or the open ends of CNT [14] . Among these functional groups, hydroxyl groups are more interesting due to the capability of converting to other organic functional groups and providing active sites for electron transfer [15] .
The feasibility of functionalization of single wall carbon nanotubes (SWCNT) has already reported in literatures. For instance, Khare et al. [16] developed hydrogenated CNTs using electric discharge to bond the oxygen containing groups such as hydroxyl and carboxyl to the wall of a CNT by treating the CNT in an oxidizing environment [17] [18] . Tian et al. reported an electrophilic addition reaction for the functionalization of SWCNT using alcohols by microwave irradiation to prepare the hydroxylated SWCNT [19] . The hydroxyl functionalization involving the covalent attachment of hydroxyl groups to atoms of SWCNTs. The stability of this covalent bonding depends on the position of C atom on the surface of SWCNT [20] . The hydroxyl functionalization of various SWCNTs has already studied using first principle calculations [21] . However, the site selectivity of the hydroxyl groups on the surface on the nanotubes has not studied yet. Due to the non-equality of C atoms on the surface of CNT from the symmetric point of view, the reactivity of these atoms is not equivalent. We used density functional calculations (DFT) to study the structures of hydroxylated CNT when one hydroxyl groups bonds to C atoms in different symmetrical positions.
2. Model and Computational Methods
In this study we used a (5, 0) zigzag carbon nanotube with 60 C atoms as SWCN model. The dangling bonds of two ends were saturated by H atoms, as shown in Figure 1. Except the C atoms at terminals, there are four types of C atoms on the surface of CNT regarding to the symmetrical positions as shown in Figure 1. Hence four geometric isomers of hydroxylated CNT models are performed when onehydroxyl group bond to these C atoms.
In the first step, we optimized the geometries of these structures using the Becke’s hybrid three-parameter exchange functional and the nonlocal correlation functional of the Lee, Yang and Parr (B3LYP) method [22] . This method was chosen because it is computationally less demanding than the other approaches as regards the inclusion of electron correlation and it has shown to successfully predict a wide range of molecular properties. We also used 6-31G(d) basis sets which have the polarized basis set for the second row of the periodic table of elements and it is implemented in the Gaussian 03
![]()
Figure 1. The (5, 0) zigzag carbon nanotube with 60 C atoms used as the model of SWCNT in this study. The dangling bonds of two ends were saturated by 10 H atoms. A-D represent the different positions of surface C atoms from symmetrical point of view.
[23] package. Due to the odd numbers of electrons in the structures, we used unrestricted B3LYP (UB3LYP) method. Using this method of theory the frequency calculations were performed to find even the structures are in the true minima and to find the Gibbs free energies for further structural stability studies.
3. Results and Discussion
All structures including non-functionalized CNT are fully optimized using UB3LYP/6- 31G(d) level of theory. The optimized structures and the frontier orbitals are shown in Figure 2. Supposing the hydroxyl-functionalized CNTs are produced from the following reaction.
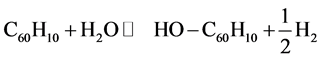
the reaction Gibbs free energy in the standard condition (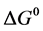 ) were obtained as:
) were obtained as:
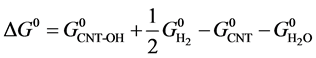
where, 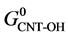 ,
, 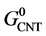 ,
, 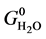 and
and 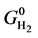 are the standard Gibbs free energies of hydroxylated CNTs, original CNT, H2O and H2 respectively, which are obtained at the theory level discussed previously.
are the standard Gibbs free energies of hydroxylated CNTs, original CNT, H2O and H2 respectively, which are obtained at the theory level discussed previously.
Last column of Table 1 represents the reaction Gibbs free energies of hydroxylated CNTs were obtained using above equation. The large variances in these values, from −24.8 to 236.5 kJ・mol−1 reveals the strong site selectivity of hydroxyl group in the reaction
![]()
Figure 2. Optimized geometries and the shapes of HOMO and LUMO molecular orbitals of 4 isomers of HO-C60H10 obtained with B3LYP/6-31G(d) level of theory. Arrows represent the direction and the size of dipole moments.
![]()
Table 1. Calculated reaction Gibbs free energies of all hydroxylated CNT as represented in Figure 1 (last column) using B3LYP/6-31G(d) theory level. Other columns represent the energy of HOMO, LUMO the band gap (ΔE) and the size of dipole moments.
with CNT. Among all structures only, structure A, which-OH group bonds to the C atom in the closest position to the terminal atoms i.e. position A in Figure 1, shows the negative value for ΔG (−24.8 kJ・mol−1). The negative value of ΔG for this structure reveals the reaction of CNT with H2O is extremely favorable with high percent of production from thermodynamic point of view. Inconsistent, the structure B with large positive value of ΔG i.e. 236.5 kJ・mol−1 is thermodynamically unstable with very low percent of production. Therefore in the production of hydroxylated CNT from reaction 1 structure A is the most abundant product whereas structures B, C and D are rare and hydroxy group like to bond with the surface C atoms which are in the neighbor of the terminal atoms of CNT.
The geometries of all HO-CNTs are highly modified regarding to the original CNT due to the bonding of OH group to the surface of CNT. Table 2 represents the C-C, bond lengths and the C-C-C angles of carbon that connected to the hydroxyl group. This information represents the order of deformation due to the interaction. The C-C bond length changes from 1.4 to 1.5 Å from the pristine nanotube to the hydroxylated one. The C-C-C angles also change from 120 to 111 degree, which reveal the Catomic S-P hybridization transform from sp2 in CNT to SP3 in hydroxylated CNT
Looking carefully to the frontier orbitals of the studied structures, which are shown in Figure 2, we realize that the shapes of the HO-CNT frontier orbitals differ to the HOMO and LUMO orbitals of CNT. Due to the covalent bonding between hydroxyl group and the C atom, π-conjugated systems in CNT break and the numbers of the π-bonds decrease in the frontier orbitals of HO-CNT with respect to the CNT especially in the positions where OH bonded to the CNT surface. This effect particularly can be seen in the structures A and D (Figure 2). Hence, the energies between HOMO and LUMO (the band gaps) increase and subsequently the electrical properties of the hydroxylated structures such as electrical conductivity change and the hydroxylated CNTs are less conductive with respect to the original one. Except the LUMO of structure D that only located on one side, othermentioned orbitals are localized on two sides of HO-CNTs.
Other molecular properties of HO-CNT such as polarization also depend on the position of -OH group on the surface. Therefore, hydroxylation would be one way of changing the electrical properties of nanotube from a conductive one to the semiconductor or even insulator CNT. The relative sizes of the dipole moments as shown in
![]()
Table 2. Geometrical parameters of optimized structures of CNTs major and minor axis’s of the cross section of the CNT inlets. α represents the inlet where is closer to −OH group and β represents the inlet which is farther from hydroxyl group. δ is the distance between the minor and the major axis. All data are reported in Å.
Figure 2 reveal the order of polarity of these structures. The size and the directions of the dipole moments also strongly depend on the positions of the hydroxyl group. The highest value for dipole moment belong to the structure D that -OH bonds to the central C atoms. Interestingly, for this structure the direction of the dipole moment vector is alongside the longitude of CNT in contradiction to our first expectation which was in perpendicular to the longitude of CNT due to the existing of more electronegative atom (oxygen) of -OH group.
The cross sections of hydroxylated CNTs are not circular. Instead they have elliptical shape. The ellipses major and minor axis of both inlets and the difference between them which are reported in Table 2 reveal the degree of deformation in CNTs due to the bonded hydroxyl group to the CNT. Structure A which is the most stable structure among the studied structures has the lower value of δ and hence it shows less deformation. It is surprising that the structure C which has the higher distance of -OH to the inlet with respect to the structure A, shows more modification on the geometry of inlets. Thus hydroxyl groups do not only modify the neighboring structure but they also represent a kind of long range interactions which are able to modify positions of atoms which are far from these groups so the whole structure are modified.
IR Spectra of Hydroxylated CNT
The calculated IR-spectra for structure A-D including pristine CNT are shown in Figure 3. This figure and the data of the strongest vibrational modes represented in Table 3 reveal that IR vibrational frequencies do not depend too much on the positions on the surface of CNT. Onlysmall shifts are seen in the O-H stretching andbending vibrational modes. The higher O-H vibrational frequency belongs to the structure A where it is the most stable structure among A-D structures. Other vibrational frequencies such as C=C stretching and bending modes do not strongly depend on the position of hydroxyl group on the surface. However, the intensities of some of these modes do depend on the position of hydroxyl group, as the dipole moments and the polarization of normal modes are not equivalent for all these structures.
4. Conclusion
In this study, the structure of different hydroxylated CNTs has been studied using
![]()
Figure 3. IR spectra of structure A-D and the CNT obtained using UB3LYP/6-31G(d) theory level. Dominated number relate to the vibrational frequencies of O-H and C-H stretching modes. The notation ν is used for stretching and δ for bending vibrational normal modes.
![]()
Table 3. The strongest vibrational mode of structure A-D and the pristine structure in cm−1. The notation ν is used for stretching and δ for bending vibrational normal modes. δ(C-H) ┴ represents the bending vibrational model of C-H groups which vibrate in perpendicular to the longitude side of CNT. * represents the most intensive frequency among the frequencies in the mentioned ranges.
UB3LYP/6-31G(d) theory level. For this purpose, a (5, 0) zigzag CNT with 60 C atoms has been used. The optimized structures of four isomers of HO-C60H10 show that the geometry and all the other molecular properties such as dipole moments, energies and the shape of frontier molecular orbitals strongly depend on the position of hydroxyl group on the surface and only one of these isomers is thermodynamically stable in STP condition. All these structure have higher band gap with respect to the pristine CNT. Thus, a hydroxylated CNT is less conductive than the pristine one.