Compressive Strength of Metakaolin-Based Geopolymers: Influence of KOH Concentration, Temperature, Time and Relative Humidity ()
1. Introduction
Inorganic materials called geopolymers (GP), which could potentially replace conventional cements and many mineral based products are becoming important in the reduction of CO2 footprint, and energy consumption. These materials have a broad application range due to their properties, such as early strength, high-temperature resistance, and acid-alkaline resistance [1] . The main factors affecting the GP properties generally depend on the utilized source, chemistry of alkaline activant, and curing conditions. A considerable number of variables or factors are involved in producing GP: most prominently precursor type and size; curing temperature and time; liquid/solid ratio; water curing regime, type and concentration of the alkali activator, the alkali activator to aluminosilicate weight ratio and so on. Investigating all of the parameters in a single work may not be possible. However, by a suitable design method, some the factors affecting these properties may be considered [2] . Besides, the effects of other main factors on compressive strength of GP have not been completely approved. While some of researches showed the increased strength with high alkaline concentration, others presented the negative impact of this on the strength. Curing at temperatures above room temperature is reported to favor the development of high compressive strength [3] - [8] ; though other authors report better results at room temperature [9] [10] , and others establish a threshold value above 60˚C - 70˚C, which would be detrimental for long term strength of GP [11] . Long curing times at relatively low temperature allow the formation of fairly homogeneous samples with highly mechanical strength, whereas breaks of the granular structure of geopolymers are observed at high temperatures [12] . Some efforts have been made to determine the influence of relative humidity on the mechanical strength. However, studies controlling relative humidity on samples are scarce and this is a key factor, since the fact that humid atmosphere completes the curing process has been proved [6] - [13] .
An experiment design method is utilized to investigate the parameters of a specific problem. Also, design method shave been applied to consider the factors affecting the properties and to decrease the number of tests, in order to consider the effects of the maximum possible factors as in the fly ash-based GP and fly ash/MK-based GP [8] [9] [14] - [19] . Experiment design in the MK-based GP was developed in systems activated with NaOH or NaOH/sodium salts [1] [20] [21] [22] [23] [24] , where compressive strength values vary largely from 7 to 75 MPa, and results published cannot be easily compared due to differences in precursors (high degree or low degree kaolin; MK obtained from kaolin at different temperatures); in high or low alkaline activators concentration, and in the large number of processing factors that are different from study to study [20] . Hydroxide solutions are activators commonly used in the synthesis of geopolymers. The precursor solution is function of the alkali metal used; therefore, the basicity and the degree of hydration of the cation are determinant in the dissolution of the aluminosilicate source. In the case of MK activated with KOH, the experimental design, the parameters affecting, and the prediction of the mechanical properties are scarce or there is none. Since the large size of K+ in this system favored the formation of bigger silicate oligomers, matrixes exhibited higher compressive strength and a specific surface area; plus a lower degree of crystallinity and resistance to attack by HCl [11] [25] .
In summary, literature offers only a small number of studies on the effects of factors on compressive strength of GP obtained by MK and scarce or none with KOH as an alkaline activator. Therefore, this study focused on the development of a suitable statistical design to investigate the effects of the parameters simultaneously by the minimum required experiment. The experimental design uses a factorial 2K + 2 to improve the performance of the parameters, such as an alkaline activator concentration, and curing conditions as relative humidity, temperature and time on the compressive strength of MK-based GP.
2. Materials and Experimental Procedure
The procedure to study the alkaline activation of metakaolin with potassium hydroxide, by an experimental design factorial 2K + 2, was divided in Design of Experiment (DOE) and statistical analysis; synthesis of geopolymers, and XRD and SEM characterization.
2.1. Synthesis of Geopolymers
Metakaolin, MK, was obtained by calcination of kaolinite, K, at 800˚C for 3 h. The chemical composition of K (80%, Fluka Analytical, particle size of 0.38 μm) is shown in Table 1, with a molar relationship Si/Al of 1.15. Geopolymer samples were synthesized by mechanically mixing stoichiometric amounts of MK and a potassium alkaline solution (87.8%, Mallinckrodt) in an electric mechanical mill (Fritsh Pulverisette 2), stirred for 15 min, to form a homogeneous slurry. Then, this fresh paste was cast into PVC cylindrical molds (13 mm × 26 mm), to shake them in a vortex (Glas-Rol, 1500 rpm), for a further 15 min to remove entrained air. The specimens were pre cured at 25˚C ± 2˚C for 96 h; removed from the molds; and finally cured in a climatic camera (HINOTEK LHS-100CH) at different times, temperatures and relative humidity.
Compressive tests were performed according ASTM C109 [26] . Three samples of each formulation were tested and the average data were recorded. A material testing machine with a rate variable (0 - 50.5 mm/min, HUMBOLDT HM-2800), was used for the measurements. The displacement was controlled at a constant rate of 0.33 mm/min for all tests.
2.2. Analysis Techniques
Fragments obtained after the resistance test were selected for characterization by scanning electron microscopy and X ray diffraction. SEM analysis was performed in a JEOL JSM 5600LV with Thermo Scientific X-ray Detector at 20 KV for chemical analysis (SEM/ EDX). X-ray powder patterns recorded on Bruker D8 Advance diffractometer, with a Brägg Brentano geometry, with Ni-filtered CuKα radiation of 1.5406 Å, at 40 KV, 30 mA, and scanning rate of 1˚ per min from 5˚ to 60˚ of 2θ, and steps of 0.02˚. The data were collected with Diffrac., Suite Evaluation, XRD Software.
![]()
Table 1. Chemical composition of kaolinite clay used, from X-ray fluorescence analysis (Spectrometer Rigaku Primus II).
aAnalysis in Wt%, bLOI: loss of ignition at 1000˚C.
2.3. Experimental Design and Statistical Analysis
In order to determine the influence of componential factors and their possible interactions in the compression strength in the MK-based GP, a factorial experiment design 2K + 2 was carried out. A total of 18 experiments were manufactured, in which various factors were changed (independent variables). The response factor (dependent variable) considered in the experiment was the compressive strength at 7 and 28 days.
The statistical significance of each single factor and their interactions, were quantitatively determined by means of an analysis of variance (ANOVA). The F-test and the p-value on the ANOVA data statistically, was considered statistically important on the response factor with a 95% confidence level. Figure 1 shows a flowchart of the methodology of the statistical analysis and multiple regression analysis.
The method called design 2K + 2 was developed to define the number of experiments N, with the Equation (1):
N = 2K + nc. (1)
where K is the number of factors and nc is the number of repetitions of the experiments in the central point. In this study, four factors are taken as independent variables: A, the activator concentration; B, the curing temperature; C, the curing relative humidity and D, the curing time. Table 2 summarizes the number of levels and the real and codified values to each factor to evaluate.
The combination of each factor level was determined with the software Statgraphics Centurion XVI®. The experiments were carried out in a random order to calculate the experimental error, according the experimental matrix (Table 3). Each factor, the statistical significance and their possible interactions amongst them on compressive strength were determined through the analysis of the variance, with the following mathematical model, Equation (2):
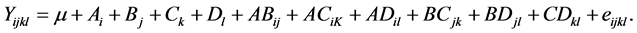 (2)
(2)
![]()
Figure 1. Flowchart of the methodology of statistical analysis.
![]()
Table 2. Factors and levels considered.
![]()
Table 3. Suggested test from full factorial method for K equal to four factors and compressive strength values.
where Yijkl is the value of the response factor (compress strength) esteemed in i, j, k and l; μ is the overall average response factor; Ai, Bj, Ck and Dl correspond to the individual effects of the factors; ABiJ, ACiK, ADil, BCjk, BDjl and CDkl represent the effect of the factors interaction, and eijkl is the random error associated with the combination of the mathematical treatments.
The F-ANOVA statistically defines the relative importance of each factor. A p-value, less than a critical level α equal 0.05, indicates that the corresponding effect of this factor is statistically significant.
3. Results and Discussion
3.1. Experimental Design 2K + 2 and Statistical Analysis
The results of the experimental design 2k + 2, factorial on the compressive strength of GP, are displayed in Table 3 and Figure 2. Derived from the eighteen experiments displayed in Figure 2, it is noted that, the compressive strength values range in a wide interval from 2.8 to 20.0 MPa. Five different conditions were found for superior strength of 10 MPa. Moreover, the geopolymerization conditions to develop a GP with the compressive strength at a higher value to 20.0 ± 0.3 MPa are those of the RO 10, in Figure 2, with 12 M KOH to 60˚C and 85% RH at 28 curing days.
Results in Table 3 were statistically analyzed, in order to investigate the significance of each factor, combination of factors and their interaction, over the compressive strength development of GP, and the validity of model. The quantitative determinations were by means of an analysis of variance (ANOVA).
The ANOVA significance analysis of the estimated effects and interactions, as well as the standard error using seven degrees of freedom is shown in Table 4. With a reliability level of 95%, and p-value less than 0.05 indicates that the corresponding effects are statistically significant at the response factor or compressive strength.
Statistical analysis results shows that the order of factors controlling the compressive strength development, in order of statistical importance, were, the activator KOH concentration > curing % RH > curing time > curing temperature (A > C > D > B). The adjusted model to an R2 at 0.9302 indicates that the 93.02% of data are explained at a 95% reliability level. The fitting goodness was evaluated by adjusted-R2 which was close
![]()
Figure 2. Compressive strength of the geopolymers specimens.
to 0.8303. Noting that 83.03% of the model explained the variability of the compressive strength, this value represents a good fit, so the model is considered suitable for further optimization [27] [28] . The p-value was greater than 0.05, which implies that there is not a serial autocorrelation on the residues (Figure 3). The mathematical model which represents the data of the statistical results is written in its actual values as follows, Equation (3):
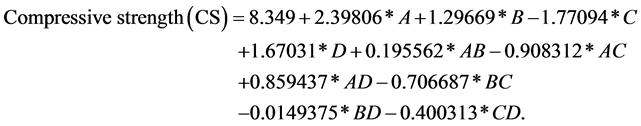 (3)
(3)
![]()
Table 4. ANOVA analysis results for compressive strength of MK-based GP (R2 = 0.9302).
SC = Sum of squares; QM = Quadratic mean; Fcal. = F-Ratio; p = p-value; e = Experimental error.
![]()
Figure 3. Normal probability plot of the residuals for compressive strength.
The response surface (RS) uses a sequence of designing experiments to develop, improve, and optimize processes. In this case, the RS was calculated on the first order as shown in Figure 4. The response surface plot, RSP, and contour plot are the visualization on the predicted model equation.
The RSP is the theoretical three-dimensional surface plot (Figure 4(a)), and the contour plot is the two-dimensional (Figure 4(b)). This plot, first of all, represents the lines of constant response which are drawn in the plane of the independent factors. The arrow indicates the direction or scaling route, which indicates the strength performance in relation to the concentration increase and the % RH decrease, keeping the curing temperature and curing time at their highest levels (B and D equals 1). On the other hand, the color bands in the contour plot denote the compressive strength estimated in a determined range, as resulting of the combination of the concentrations and the %
![]()
![]()
Figure 4. (a) Response surface chart and (b) Contour plot on the compressive strength for the MK-based GP.
RH. These plots give useful information about the model fitted, but they may not represent the true behavior of the system [29] (Figure 4).
3.2. Effect of Factors on Compressive Strength of GP
The amount of this constant may be important, so the average values are to be used. This study of factors only shows the effects of factors on compressive strength. To analyze this effect, minimum and maximum values of a specific factor are used [8] . The influence of factors is described of statistical significance.
3.2.1. Activator Concentration [KOH]: Factor A
The influence of the activator concentration on the compressive strength performance (Figure 5) shows that as KOH increases, strength rises. This positive response allows increasing gradually the GP strength, and this is in function of time, temperature, and % RH of the curing processes. A good example of this takes place when the concentration is 8 M KOH with curing conditions of 60˚C and 85% HR at 28 days, the compressive strength is 8.2 MPa, and with 12 M KOH, at the same curing conditions, the strength improves to its highest value of 20.0 MPa, signifying a 58.77% growth. When the concentration goes from 8 to 12 M KOH, with different curing conditions of 40˚C and 95% HR at 7 days, the strength only increases in 52.53%. When the concentration of the activator increases, not only does it produce a rise in the strength, but it also leads to the formation of carbonated species in function of the curing conditions.
Results of the influence of the activator concentration on the compressive strength performance (Figure 5), match those of other authors where the concentration of activator (NaOH/Na2SiO3, Ca(OH)2) by DOE is one of the principal factors on strength of
![]()
Figure 5. Influence of the concentration of KOH on the compressive strength development.
GP obtained by MK or other precursors as fly ash and slags [1] [18] [30] . Additionally, these confirm that there should be an optimum ratio to have the highest strength [8] [31] [32] . In this case, where KOH has been found, the K+ by binding to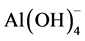 , favors the formation of larger silicate oligomers. Therefore, the geopolymer results in better setting and stronger compressive strength compared to geopolymers synthesized in NaOH [33] .
, favors the formation of larger silicate oligomers. Therefore, the geopolymer results in better setting and stronger compressive strength compared to geopolymers synthesized in NaOH [33] .
3.2.2. Percent Relative Humidity, % RH: Factor C
The relative humidity, in the curing process, plays key roles in the development of the GP microstructure and properties [6] [34] [35] [36] . In the present work, the % RH is a significant statistical factor with a negative effect on the compression strength (Figure 6), since samples exposed to atmospheric carbonation, and high alkaline activator concentration, induce a loss of mechanical strength.
Thus, % RH is a key factor in the carbonation process of the geopolymerization, where an intermediate humidity is required to enable an uptake of CO2 from the atmosphere, which is slow under either very dry or saturated conditions [37] . The analysis trend of GP compressive strength regarding the relative humidity percentage at different curing times and activator concentration, in Figure 6, indicates that with an increase in % RH, > 95%, tends to decrease the strength and the activator concentration. Due to the CO2 evolution in the geopolymerization for GP of RO 18 (12 M, 60˚C, 85% HR, 7 days), at early stages and high alkali concentration, carbonation occurs rapidly. Then, with the development of geopolymerization (RO 10: 12 M, 60˚C, 85% HR, 28
![]()
Figure 6. Influence of the percent relative humidity on the compressive strength development (RT = Room temperature).
days), the sample cured, becomes compact, which hinders the absorption of CO2 by matrix, and formation of carbonates (Figure 7).
Accelerated carbonation tests used relative humidities between 50% - 70%, where concretes and alkali activated slags were carbonated at the fastest rates. With relative humidity values of over 80%, the pores became waterlogged and impeded the CO2 dissolution [37] [38] . The effect in the geopolymerization with 85% RH is adequate in decreases of carbonation; while a carbonation increase happens at 95% RH.
3.2.3. SEM Morphology
Micrographs analyses by SEM-EDX were performed, for GP of RO 10 y 18 respectively, at the same curing conditions, except time. Micrographs, after the strength test, showed an amorphous microstructure with pores, porosity, and cracks at 7 curing days (Figure 7(a)). The presence of pores and porosity is related to the fast water evaporation, air trapped in while these gradually decrease until 28 days, producing a compact GP, without porosity or cracks (Figure 7(b)); due to gradual water expulsion. This suggests a progression of reaction [39] .
3.2.4. Curing Time: Factor D
Curing time has a considerable impact on mechanical strength development [38] [40] . The evolution of geopolymerization, for samples RO 10 and RO 15 selected due to their
![]()
Figure 7. Representative scanning electronic micrographs of the geopolymers (a) RO 18 and (b) RO 10.
high and low strength, respectively, are presented at 7, 28, 60 and 90 curing days (Figure 8 and Figure 9). In this, curing time condition has a significant and positive effect on developing microstructure and mechanical strength.
![]()
Figure 8. Influence of the curing time on the compressive strength development to RO 10.
![]()
Figure 9. Influence of the curing time on the compressive strength development to RO 15.
The evolution of the GP, RO 10, is followed by surface micrographs in Figure 8. Initially, at 7 curing days the surface seems an amorphous matrix with isolated pores of sizes less than 2 μm. Evolution of the matrix at 28 days shows a compact and smooth homogeneous surface, without pores or cracks, with tiny plates MK embedded in the matrix. From 60 to 90 curing days an amorphous compact and homogeneous material is consolidated, with a progression in strength from 33.5 to 42.0 MPa, respectively. In the surface, during geopolymerization, the MK plates embedded, suggest that either these serve filling in gaps left in the matrix or allow gradual precipitation of gel on plates MK, covering them, which leaves a homogeneous surface, reducing the pores and increasing strength [25] [41] [42] .
The evolution of the sample with the lowest strength, RO 15 initially presented a heterogeneous surface at 7 curing days (Figure 9), with irregular particles of long size less of 2 μm and pore sized less than 2.5 and 5 μm. At 28 curing days, large agglomerates were formed (˃5 μm), with pores (˂2 μm). Later, at 60 days, the surface appears amor- phous and homogeneous with isolated pores, suggesting matrix evolution until this time. Finally, at 90 curing days, the surface is consolidated compact and amorphous without pores. Thus, GP acquires an increasingly compressive strength of 58.6% from 5.8 MPa to 14.0 MPa, since 28 to 90 days, respectively.
The time curing results match those of other studies, where the activation of precursor needed time for the geopolymerization to occur was observed, thus requiring a period of time in order to increase the extent of the process for strength gain. The desired curing temperature and time where the compressive strength acquired day by day indicating that geopolymerization took place continually without deterioration at later times [42] . At ambient temperature, the reaction is extremely slow [43] [44] . When the sample cured at high temperature for a longer time, it caused the occurrence of gel contraction, loss of water molecules, and shrinkage [43] . But, when curing at moderate temperatures (40˚C and 60˚C) and times of 28 and 60 days, it showed a slightly faster strength gain without dropping at longer ageing time [43] [45] [46] .
3.2.5. Curing Temperature: Factor B
The compressive strength of the GP, with variation of alkaline concentration and % RH in function of temperature, in Figure 10, indicates that at 60˚C at 7 and 28 days, improves at 60˚C, compared to that at room temperature (23˚C ± 3˚C) and 40˚C. Samples with raising temperature from 40˚C to 60˚C, activated with 12 M KOH and 85% RH, increase strength, from 13.4 to 20.0 MPa, at 28 curing days. In contrast, samples with the same temperature raise, activated with 12 M KOH to 95% RH, and, 8 M to 95% RH, whose strength values were insignificant, at 7 and 28 days.
These results suggest that curing temperature at 60˚C, has a significant influence on compressive strength development since that promotes the dissolution of the precursor (MK) and continuous geopolymerization; particularly at early stages (7 days), unlike at room temperature that seems to have no effect on the development of strength (<4 MPa). Thus, the strength at 40˚C and 85% RH curing conditions needs 28 days to
![]()
![]()
Figure 10. Influence of the curing temperature on the compressive strength development (a) 85% HR and (b) 95% HR.
develop a similar value at 60˚C and 85% RH. For this reason, 85% RH is set as optimum at 60˚C in this work.
The more curing temperature increased, the more compression strength did also, with agreeing results regarding those from other works [1] [7] [8] [47] - [53] , which reported that using curing temperatures between 40˚C and 80˚C between 4 and 48 hours is one of the important conditions for the synthesis of GP [46] . Some researchers found that the curing temperature was a reaction accelerator in fly ash-based GP. Heat curing was also required to produce a fast geopolymerization process to achieve an acceptable strength within very short periods [7] [45] [47] [51] , but others mentioned that a higher curing temperature does not necessarily ensure that the compressive strength of the product will be higher [7] [50] [52] . Therefore, the geopolymer reaction requires the presence of moisture in order to develop good strength, similar to that found in the results of this study (60˚C and 85% RH). Compressive strength increased with the moderate elevation of the reaction temperature.
3.2.6. Powder X-Ray Diffraction
The XRD of geopolymer of the samples RO 10, RO 15 and RO 18 are shown in Figure 11. The main component of the GP was the broad and amorphous hump, characteristic of geopolymeric reactions; with minor unreacted quantity of quartz, illite and microcline from MK precursor, and kalcinite carbonated phase, its formation depends on the concentration and curing conditions. The initial halo of MK from 15˚ - 35˚ 2θ with KOH was modified up to 20˚ - 40˚ 2θ, indicating the production of the gel of aluminosilicate [54] .
![]()
Figure 11. XRD patterns of the samples (a) RO 15, (b) RO 18, (c) RO 10, (d) MK and (e) K.
4. Conclusions
The design of experiments based on the 2K + 2 model was proposed to study the influence of four parameters: concentration, time, temperature and % RH on the synthesis of GP by MK activated with KOH. The following remarks were derived from the results in this study:
・ The compressive strength of 18 samples with different ratios was tested, the results indicated a yield between 2.8 ± 0.5 and 20.0 ± 0.3 MPa.
・ The statistical analysis results reveal that the ordering of the factors involved in the strength of geopolymers is, according to their importance: the concentration of the activator KOH > curing % RH > curing time > curing temperature.
・ The % RH had a significant negative influence on the strength of GP, due to the carbonates formation, in contrast with the rest of the factors which showed a significant positive effect.
・ The study exposed that the control of % RH decreased carbonation by atmospheric conditions. Thereafter, a MK-based GP prepared with 12 M KOH curing of 85% RH and 60˚C at 28 days was favorable for a high strength of 20.0 ± 0.3 MPa. Moreover, from 28 to 90 days the maximum increase in compressive strength (20.0 to 42.0 ± 0.4 MPa) was obtained.
・ The highest compressive strength achieved of 20.0 ± 0.3 MPa (12 M KOH, 85% RH and 60˚C) at 28 curing days was regarded as fully reacted GP cement. SEM analysis proved the formation of gel binder of this geopolymer.
The research carried out in this study has shown the importance to design of experiments in developed geopolymers with a homogeneous and compact structure with high compression strength. MK-based GP has shown potential in construction and environmental engineering applications.
Therefore, it deserves more efforts to investigate the precursors, in order to work with low grade kaolinite to reduce costs. The use of other activators such as KOH/ K2SiO3 or KOH/Na2SiO3 could improve the setting time, flowability, and strength development of geopolymers to continue the DOE 2K on response surface, along with the optimization and prediction of the process.
Acknowledgements
Acknowledged financial support includes the Universidad Nacional Autónoma de México, UNAM (PAPIIT, Project No. IN 114412), the Facultad de Química, UNAM (PAIP, Project No. 5000 9038), and CONACYT PhD Scholarship to Tania A. García Mejía (CVU 334206). The authors wish to thank Dr. Raúl Herrera Becerra (Instituto de Física, UNAM), Dra. Rosa María Ramírez Zamora (Instituto de Ingeniería, UNAM) for his support and Professor Miguel Ángel Hernández Alcántara (Facultad de Ingeniería, UNAM) by the design and manufacture of PVC molds. Dr. Jesús A. Arenas Alatorre and M. en C. Ma. Cristina Zorrilla Cangas are acknowledgment by their help in the SEM analysis (Laboratorio Central de Microscopía, IF-UNAM). The authors are grateful to Dra. Teresa Pi Puig, M. en C. Ma. Cecilia Salcedo and M. en C. Adriana Tejeda Cruz for the XRD measurements.