Brain MRI Findings in Infantile Spasm: Outcome Correlations in a Patient Cohort ()
1. Introduction
Infantile spasm is a specific type of seizure that is seen in infancy and childhood [1] with an incidence rate of 2 - 5 cases per 10,000 live births [2] - [5] . It is defined as an abrupt, often clustered, extension-flexion or mixed extension-flexion movement of primarily the proximal and truncal muscles [6] . West syndrome is an epileptic syndrome which presents with infantile spasms. The criteria to diagnose West syndrome includes: i) developmental plateau or regression [7] [8] ; ii) seizure type (epileptic spasm) and iii) hypsarrhythmia pattern on electroencephalography (EEG) [9] . The great majority of patients with infantile spasms will also have developmental problems and hypsarrhythmia on EEG, and therefore patients with West syndrome or just infantile spasms tend to be reported as one group [10] [11] . This is the group in our study.
The prognosis of infantile spasm is multifactorial and depends on: i) the underlying cause, i.e. congenital brain malformation; ii) the pattern of electroencephalography; iii) the appearance of seizures prior to the spasms; and iv) a rapid response to treatment [12] .
Current classification of infantile spasm refers to genetic, structural-metabolic, and unknown cause [13] , replacing the previous use of idiopathic, symptomatic, and cryptogenic respectively. Etiologic classification of infantile spasm has shown structural- metabolic abnormalities in 68% of children, unknown etiology in 24% of children and genetic causes in 8% of children [14] . MRI can provide information regarding etiology, thus can aid in the categorization of infantile spasm [15] .
The purpose of this study was to compare MRI findings with neurodevelopmental outcomes in children with infantile spasm where preliminary clinical work up did not lead to a specific etiological diagnosis.
2. Materials and Methods
2.1. Subjects
This retrospective review included the clinical charts of 26 infants with infantile spasm that underwent brain MRI and clinical follow-up between December 2007 and February 2014. These cases were extracted from the hospital medical records of 400 children with seizures admitted during that period. Inclusion criteria included; a) infants with clinical evidence of infantile spasm; b) proven by hypsarrhythmia or modified hypsarrhythmia on electroencephalogram EEG after neonatal period to 12 months of age. Children were excluded if: a) other types of seizures were encountered, b) if clinical and EEG findings were not supportive of infantile spasm, or c) if images were of poor quality. Prior to evaluation of the MRI findings, the cases were classified as structural-metabolic, genetic, or infantile spasm of unknown etiology based on clinical information, EEG and laboratory tests.
2.2. MRI Technique and Interpretation
All children underwent an MRI scan on a 1.5 Tesla clinical MR system (Signa HD, General Electric Healthcare Technologies, Waukesha, WI, USA). All children were given anesthesia which was administered by a pediatric anesthesiologist as per departmental protocol. Images were acquired using an 8-channel head coil. All of the MRI’s included the following sequences (time of repetition/time of echo/thickness/field of view): Axial T1 Fluid-Attenuation Inversion Recovery (9502/125/5/352 × 224), axial T2 Fast Spin Echo (FSE) (2221/26/5/320 × 256), coronal T2 FSE (5000/101/5/320 × 256), fast relaxation fast spin echo (frFSE) (4650/102/5/320 × 256), sagittal T1 Fluid-Attenuation Inversion Recovery (2100/24/5/320 × 256), Diffusion Weighted Images (DWI) (8000/72/5/128 × 128), and coronal Fast Spoiled Gradient-Recalled brain volume imaging (FSPGR BRAVO) (Flip angle 14/TI10.4/thickness1.2/320 × 320). Susceptibility weighted angiography imaging (SWAN) (78.3/50/10/288/224) was done on patients who were imaged in 2010 and thereafter.
2.3. Data Collection
Medical records were systematically reviewed. Data regarding gestational age at birth, the age at first spasm, course of the disease, treatment history, time under follow-up and the outcome of the patient was reviewed. MR images were reviewed by a pediatric radiology fellow (AK) who was blinded to the underlying diseases, clinical history and outcome. Image findings were compared to the clinical report in the Picture Archiving and Communication System PACS. The median follow-up period for infantile spasm patients was 29 months (range 1 - 66.7 months).
The diagnosis of infantile spasm and the EEG findings were confirmed by a pediatric neurologist with 7 years of experience in pediatric neurology (ES).
All patients received therapeutic regimen including Vigabatrin and/or adrenocorticotrophic hormone ACTH.
The initial classification of infantile spasm was correlated to the MRI findings and final diagnosis, as documented in clinical follow-up records, to evaluate the usefulness of MRI in the categorization of infantile spasm. Finally, the neurodevelopmental outcome was evaluated according to response to treatment for spasms and seizure control and the presence of any degree of developmental delay/regression for each group.
Children with no or mild developmental delay, without spasm or other seizure activities were categorized as favorable neurodevelopmental outcome while children with moderate-severe developmental delay and with other seizure activities and/or spasm were categorized as unfavourable neurodevelopmental outcome. The degree of developmental delay (mild, moderate, severe) was extracted from the chart information as per clinicians’ decision. Data was analyzed using a two tailed Fisher exact test for any correlations and the p-values were calculated.
3. Results
The age of initial infantile spasm onset ranged between 2 - 11.5 months (mean 6.4 months). The mean onset age was 6.6 months (5 - 9 months) for infantile spasm of unknown etiology, and 6 months (2 - 11.5 months) for genetic/structural-metabolic infantile spasm. Clinical follow-up time ranged between 1 - 66.7 months (mean 29 months). Demographics are summarized in Table 1.
3.1. Value of Neuroimaging for the Clinical Diagnosis
MRI was supportive in the classification of 14/26 children with infantile spasm, changing the diagnosis in 8/16 children with infantile spasm of unknown etiology to structural-metabolic group (p-value = 0.0256).
Based on clinical information, EEG, and laboratory tests, 10/26 (38.46%) structural- metabolic or genetic group (including sequelae of hypoxic-ischemic encephalopathy and meningitis) were diagnosed prior to imaging. In 6/10 (60%) cases, MRI revealed abnormalities in support of the diagnosis and in the other 4/10 (40%) cases, MRI did not add additional information (2 had non-specific MRI changes and 2 had a normal MRI; 3/4 of these cases had chromosomal abnormalities).
Unlike the above group, MRI was helpful in the further classification of the remaining 16/26 (61.54%) children with infantile spasm who had no identifiable cause, changing the diagnosis in 8/16 children with clinically unknown infantile spasm to structural- metabolic while the remaining 8/16 children stayed as unknown group (p-value = 0.02).
Final classification of this cohort resulted in 18/26 (69.23%) children with genetic/structural-metabolic infantile spasm and 8/26 (30.77%) with unknown etiology infantile spasm based on combination of clinical, laboratory and neuroimaging findings (Diagrams 1 (a)-(c), Figure 1 and Figure 2).
![]()
Table 1. Demographics of infantile spasm cases in either category; gestational age, age at first spasm, duration of follow-up and neurodevelopmental outcome.
†Case no. 19 died one month after starting treatment due to Leigh disease. M = male; F = female; m = month; d = day.
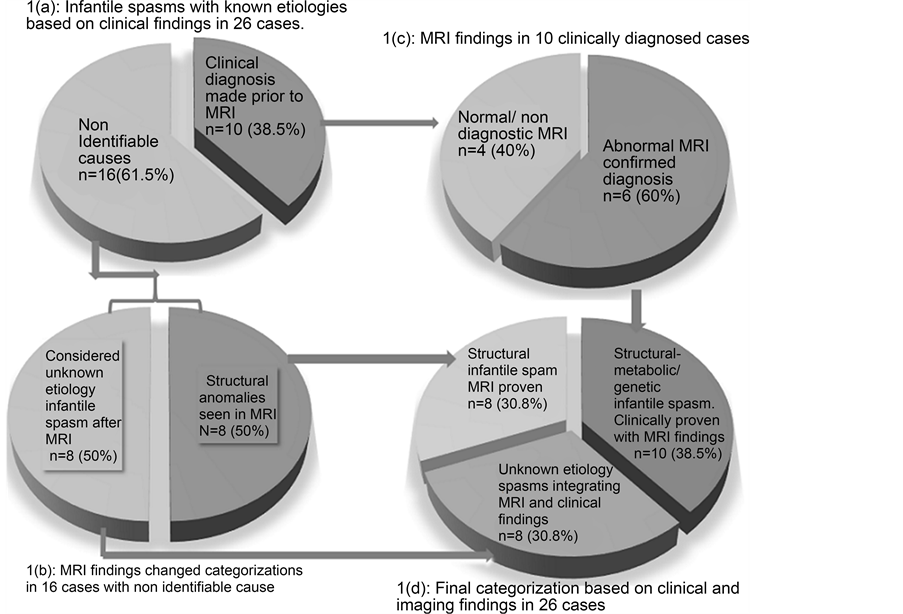
Diagram 1. Infantile spasm categorization: (a) based on clinical diagnosis; (b) & (c) integrating MRI findings for further categorization; (d) final categories of infantile spasm in the cohort.
![]()
Figure 1. A 6-month-old baby boy with Miller-Dieker syndrome (case no. 13). No definitive etiology before imaging. Axial (a, b) and coronal (c). T2 weighted images demonstrate lissencephaly agyria/pachygyria and thickened, band-like heterotopia in subcortical white matter known as double-cortex (arrows). The thickness of the band heterotopia is marked with a brace in the image (c). The image findings assisted in coming to a final diagnosis for this child.
3.2. Imaging Findings of Infantile Spasm
The etiologic causes in the structural-metabolic or genetic group are summarized in Table 2. In 3/8 (37.5%) cases of infantile spasm of unknown etiology, nonspecific and minor imaging abnormalities were demonstrated (Table 3, cases 3, 4 and 7). On the contrary, among the 18 cases of structural-metabolic or genetic infantile spasm, 16/18
![]()
Figure 2. 2-month-old term baby girl (case no. 23) with spasms and without identifiable etiology prior to imaging. Axial T2W image (a) demonstrated foci of hypointensities on the frontal subcortical and periventricular white matter (arrows). T1W image (b) revealed hyperintensities on the same regions visualized on T2W images (arrows) due to subcortical and sub ependymal tubers of tuberous sclerosis. The patient categorization changed to structural type infantile spasm.
![]()
Table 2. Etiologic causes of structural metabolic/genetic infantile spasm.
![]()
Table 3. MRI findings in infantile spasm of unknown etiology.
N = normal; CC = corpus callosum; CSF = cerebrospinal fluid; VM = ventriculomegaly; Rvisc = reversible vigabatrin-induced signal changes; Ba = blooming artifacts. #Case 7 demonstrated a small cavernoma in the posterior fossa, unlikely related to infantile spasm.
(88.9%) demonstrated MRI abnormalities and only 2/18 (11.1%) children had normal neuroimaging. The correlation between abnormal MRI and structural-metabolic or genetic type infantile spasm was significant (p-value = 0.01). A summary of neuroimaging findings in structural-metabolic or genetic infantile spasm are found in Table 4.
3.3. Follow-Up & Outcome
Clinical follow-up ranged between 1 - 66.7 months (mean 29 months). In 17/26 (65.38%) cases, there was no spasm present in the follow-up, but other seizure activities continued in 15/26 (57.7%) cases, of which 5 (19.23%) cases had refractory seizures.
Spectrum of developmental delay was observed in 24/25 (96%) children. One child with Leigh disease deceased one month after initiation of antiepileptic therapy.
![]()
Table 4. MRI findings in structural-metabolic/genetic infantile spasm.
† IS = infantile spam; HIE = hypoxic-ischemic encephalopathy; T = thalamus; P/C = putamen/caudate; GP = globus pallidus; PV = periventricular white matter; W = white matter; P/MB = pons/midbrain; CP = cerebral peduncles; CC = corpus callosum; WV = widened ventricle; WCSF = widening of CSF space; CD = cortical dysplasia; PG-PMG/pmg = pachygyria-polymicrogyria; HT = heterotopia; T = tuber; AS/S = absent septum pellucidum/schizencephaly; M = megalencephaly; L = lissencephaly; dis = disease. †† 2 cases with trisomies 21 and 15 had normal imaging. They are not included in the table. ††† Case with trisomy 15 revealed rvisc “reversible vigabatrin-induced signal changes” and not shown in this table.
Among infantile spasm of unknown etiology, complete resolution of spasm was achieved in 7/8 (87.5%) cases; but 3 of them continued to have other seizure types (one refractory, one focal motor and one complex partial seizure). This group of infantile spasm encountered a spectrum of delay in developmental milestone achievements in 7/8 (87.5%) cases; 3 with mild delay. Only 1/8 (12.5%) case with infantile spasm of unknown etiology achieved a normal developmental milestone at 26.6 months follow-up with complete resolution of spasm and no seizure activities on anticonvulsant (Table 5).
In contrary, among the structural-metabolic and genetic infantile spasm cases, there was heterogeneity in resolution of spasm and other seizure activities. As shown in Table 5 and Table 6, spasm resolution irrespective of having other types of seizures, was observed in 10/18 (55.55%) children while seizures with/without spasm continued in 12/18 (66.67%) children; 4 of whom had refractory seizures. Only 2/18 (11.1%) children categorized as structural-metabolic and genetic infantile spasm became spasm and seizure free in follow-up.
The difference of spasm resolution between the infantile spasm of unknown etiology and structural-metabolic/genetic group was not statistically significant (p-value = 0.19). All children with structural-metabolic and genetic infantile spasm demonstrated developmental delay including regression in 2 patients (Table 6).
Among the group of infantile spasm with unknown etiology, 5/8 (62.5%) cases had favorable neurodevelopmental outcome regarding spasm resolution, discontinued seizure activity and mild or no developmental delay while only 1/18 children in the structural-metabolic or genetic cases had favorable outcome (Diagrams 2 (a)-(c)). The difference was significant (p-value = 0.004).
![]()
Table 5. Outcome of infantile spasm of unknown etiology.
If patient had spasm, seizure or developmental delay, it is marked as *. Patient with favorable neurodevelopmental outcome marked as +. M = minimal. R = refractory. F = focal. A = autism. † Based on our criteria, continued any type of seizure and/or spasm with any degree of developmental delay considered as unfavourable outcome.
![]()
Table 6. Outcomes of structural-metabolic/genetic infantile spasm.
If patient had spasm, seizure, developmental delay or favorable neurodevelopmental outcome, it is marked as *. HIE = hypoxic-ischemic encephalopathy; dys = dysplasia; con = congenital; N = not applicable (Patient deceased). Reg = regression; R = refractory; syn = syndrome. † = this patient had mild delay.
3.4. Treatment Related Imaging Abnormality
Vigabatrin-induced signal changes on MRI were seen in 2/26 cases (7.7%); both with signal abnormalities in the globus pallidi that disappeared after discontinuation of Vigabatrin (Figure 3).
4. Discussion
We targeted brain MRI and its role in the etiologic work-up and prognosis of infantile spasm of unknown etiology. Neuroimaging in our study group had substantial influence to further classify infantile spasm in patients when the preliminary clinical evaluation and work-up did not reach a specific diagnosis (16/26 (61.54%) in our cohort (p-value = 0.0256)).
In our study, MRI identified brain abnormalities in 73.1% (19/26) of cases versus 26.9% (7/26) of cases with normal imaging. This is comparable to Saltik et al. [16] who studied a series of 86 patients and found normal MRI in 22 (25.6%) cases and abnormal MRI in 64 (74.4%) cases. They found that MRI could solely reveal the underlying cause in 41.8% of cases which was similar to 30.77% (8/26) of cases in our study.
![]()
Diagram 2. Neurodevelopmental outcome of infantile spasm: (a) unknown etiology; (b) structural-metabolic/genetic; (c) cohort of infantile spasm cases.
![]()
Figure 3. 14-month-old baby girl with trisomy 15 and infantile spasm at 11 months of age (case no. 26). MRI revealed T2W (a) and FLAIR (b) hypersignal intensities in the globus pallidi and restriction in diffusion weighted images (c) (upper row arrows). MRI at the age of 22 months, 6 months after discontinuation of Vigabatrin, reveals resolution of the signal changes (lower row d, e, f).
The outcome of infantile spasm is generally poor [17] . There is a consensus that when an etiology is not identified in children with infantile spasm, a better prognosis is expected compared to the structural-metabolic type of infantile spasm [17] - [19] . Favorable neurodevelopmental outcome among our patients was achieved in a small number, 6/26 (23.1%) cases. This proportion was substantially higher among the infantile spasm of unknown etiology group, 5/8 (62.5%) children in contrast to just 1/18 (5.55%) case of favorable outcome in the structural-metabolic/genetic group (p-value = 0.004). This data is supported by Cohen et al. [18] who reported good neurodevelopmental outcome of infantile spasm of unknown etiology. Our results were comparable to Ducal et al. [20] who reported favorable outcome in 66.67% of their cryptogenic infantile spasm cases (now classified as infantile spasm of unknown etiology). Our results are also similar and supported by a meta-analysis by Widjaja et al. [17] that reviewed the outcome of 2967 infantile spasm cases and a positive outcome was achieved in 26.4% of cases (95% CI = 0.197 - 0.344). According to this study, cryptogenic (now classified as unknown etiology) cases had a good outcome in 54.3% of cases (95% CI = 0.458 - 0.625) versus symptomatic (structural-metabolic/genetic) infantile spasm that had a good outcome in 12.5% of cases (95% CI = 0.09 - 0.171).
The underlying cause of brain abnormalities in our structural-metabolic or genetic group was similar to a larger series by Wheless et al. [21] including: cerebral dysgenesis in 25.4% of cases, tuberous sclerosis complex (TSC) in 13.7% of cases, hypoxic-ischemic encephalopathy (HIE) in 8.8% of cases and chromosomal anomaly in 8.8% of cases.
Our data indicated that normal imaging was rare in structural-metabolic/genetic infantile spasm and if present, did not carry any predictive value regarding better outcomes compared to structural-metabolic/genetic infantile spasm with abnormal MRI. Being aware of the underlying etiology is possibly the most important criterion to predict the outcome of children with infantile spasm. As established in previous studies [22] [23] , infantile spasm of unknown etiology revealed normal MRI or non-specific changes and carried favorable outcomes when compared to structural-metabolic/genetic infantile spasm in our study.
The limitations of this study include the small sample size, heterogeneity of the cases, and the length of time that the cases were followed. It also suffers from lack of specific developmental testing because it is a retrospective study and as a result the neurodevelopmental outcome is evaluated on the data in the patient’s clinical chart.
5. Conclusion
In summary, i) MRI has a well-established role in identifying the cause of infantile spasm, adding information in 1/3 cases with previously unknown etiology; ii) non-specific findings on MRI follow the same outcome as normal MRI in our cohort; and iii) resolution of spasm is independent of MRI findings, therefore MRI cannot predict spasm medication response.
Declaration of Conflict of Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed no financial support for the research, authorship, and/or publication of this article.
Acknowledgements
We thank Mrs. Cassandra Kapoor, research administrator at Children’s Hospital of Eastern Ontario for the administrative and editorial support.
Author Contributions
AK contributed in review articles, select patients, data acquisition, interpretation, data analysis, statistics, art works and writing manuscript. MA contributed in review articles and select patients. ES contributed in select cases, clinical interpretation, manuscript writing and manuscript revision. EM supervised the research and contributed in image analysis and interpretation, review articles, critical manuscript revisions and manuscript writing.
Ethical Approval
The Children’s Hospital of Eastern Ontario Research Ethics Board approved this study.