Optimization Studies for Selective Recovery of Phenolics from Wine Wastes ()
Received 4 March 2016; accepted 15 April 2016; published 19 April 2016

1. Introduction
In the last few years diminishing the environmental impact of industrial wastes has been a subject of increasing concern. Grapes are one of the world’s largest fruit crops and wine-making wastes are rich in phenols. These and other organic compounds also contained considerably increase biochemical and chemical oxygen demands, with detrimental effects on the flora and fauna of discharge zones, while in solid residues used as fertilizers, they may inhibit germination properties. On the other hand, grapes, wine, grape seeds and skins extracts are reported to exert favourable effects on human health attributed to their phenolic content [1] - [4] .
Literature is rich of examples of recovery of antioxidant compounds from natural sources such as oil seeds, nuts, vegetables, fruits, etc. [5] . Phenolics present antioxidant activity and thus are considered as high added- value by-products and the employment of low-cost industrial wastes could greatly reduce the production costs and increase the margin profit of these products [6] . Most of the published work referred to phenolics from the whole fresh grapes [2] [7] , whereas recent ones, but few, dealt with valorization of wine wastes and their phenolic content [8] [9] . Based on the above, we undertook an investigation on the recovery of specific bioactive phenolics from grape wastes, which were produced in large amounts in wineries all over Greece.
Solvent extraction methods followed by sorption on specific sorbent materials were chosen. The parameters which influenced the yield of phenolics extraction from wine wastes, such as the solvent type, solid to solvent ratio, pH value, extraction time and temperature were studied, applying One-Factor-at-a-Time (OFAT) experiments each time while keeping others fixed.
When many factors and interactions affect desired parameter, response surface methodology (RSM) is an effective tool for optimizing the process [10] . RSM is one of the most commonly used Design of Experiments (DoE) technique for the optimization of complex processes. It uses quantitative data from an appropriate experimental design to determine and simultaneously solve multivariate equation. Central Composite Design (CCD) is a widely used response surface design when the experimental region is defined by the upper and lower limits of each factor and not extended beyond them [11] . Optimization of solvent extraction method was also attempted in our study using RSM where the simultaneous effect of three independent variables (pH value, solid to solvent ratio, temperature) were investigated to maximize the recovery of phenolics and their antioxidant activity.
Although organic solvents are useful for the extraction of metabolites from plants, further purification, in order to selectively obtain concentrated specific components, can be essential. Adsorption is preferred by many researchers among others as a low-cost separation technique [12] . Various sorbent materials have been used for selective separation of phenolics, such as resins or activated carbon forms from several sources such as spinach, apple pomace or grape pomace [13] [14] . Apart from that, zeolites and alumina have been also used as sorbents [12] [15] - [18] .
The results from the optimized solvent extraction, followed by sorption and subsequent desorption regarding phenolics and their antioxidant activity, are reported here.
2. Materials and Methods
2.1. Materials
Grape marc and lees (Malagouzia, white local variety and Syrah, red variety) were kindly provided by “Ktima Gerovassiliou”, a wine-making factory in Epanomi (Thessaloniki, Greece) in the vintage 2013. Grape marc samples were collected after pressing and consisted of skin and seeds mainly, whereas white lees were collected before fermentation. Samples were dried at ambient temperature in a desiccator and milled in a commercial blender (i.d. ≤1 mm).
2.2. Extraction
Grape marc and lees (100 g dry weight) were extracted with a certain volume of solvent in a sonicator bath (General sonic, 41 kHz, 320 W, thermostatically adjustable) at the temperature and the time established. The influence of various parameters (pH, temperature, solvent type, extraction time and solid to solvent ratio) on the extraction efficiency of phenolic compounds was investigated. The extracts thus obtained, here after called crude extracts, were centrifuged (4500 rpm, 10 min), stored in the refrigerator (−20˚C) and further analyzed.
2.3. Determination of Phenols, Sugar Content and Antioxidant Activity
Total phenolic content was determined using the Folin-Ciocalteu method and measuring the absorbance of the blue complex formed at 745 nm [19] . Total phenolics content (TPC) was expressed as mg of gallic acid g-1 (dry weight).
Sugars were determined photometrically by measuring the absorbance at 575 nm, using dinitrosalicylic acid method [20] .
Antioxidant activity was estimated using the 2,2-diphenyl-1-picrilhydrazyl (DPPH) method with modifications [21] . The DPPH solution (0.1 g∙L−1 in ethanol) was prepared daily, stored in a flask covered with aluminum foil and kept in the dark at 4˚C between measurements. The percent decrease in absorbance (equation 1) was recorded for each concentration and percent quenching of DPPH radical was calculated on the basis of the observed decrease in absorbance of the radical.
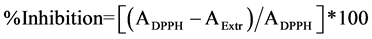 (1)
(1)
where ADPPH is the absorbance value of the DPPH blank sample and AExtr is the absorbance value of the test solution.
2.4. Sorbents
The sorption of phenolic compounds from wine wastes samples was carried out using different organic and inorganic materials, namely, aluminum oxide, zeolite, ash, agar, activated carbon, resins XAD-2, XAD-7 and XAD-16 (Sigma Chemicals). The clinoptilolite-rich mineral (zeolite) (particle size 200 μm) was obtained from Metaxades, Thessaloniki. Treatment of zeolitic samples was carried out using 2 M NaOH under continuous stirring for 24 h. After that, the solid phase was separated by filtration, washed with deionised water and dried in an oven at 60˚C over night. Aluminum oxide was stirred with pure methanol for 20 min, centrifuged (4500 rpm, 10 min) and dried over night in a desiccator before use.
2.5. Sorption/Desorption Experiments
Batch sorption experiments were conducted by stirring 0.02 L of extract from marc or lees samples with 0.5 g of sorbent at ambient temperature. The effect of contact time (up to 2 h), pH (2.0 to 10.0) and sorbent concentration (5, 10, 19, 25 and 50 g∙L−1) was examined. Samples were centrifuged (4500 rpm, 10 min) and the supernatant was analyzed for phenolics, radical scavenging activity and sugar content.
In case of zeolite and aluminium oxide, another series of sorption experiments were conducted, using pre-treatment zeolitic and of aluminum oxide samples as described above.
Desorption experiments, were conducted with 0.1 M HCl. The effect of contact time was examined here also (up to 2 h) and the desorbed phenolics, their radical scavenging activity (DPPH inhibition %) was determined as previously described.
2.6. Experimental Design Applied to Extraction Procedure and Statistical Analysis
The individual and interactive effects of the mass to solvent ratio (0.0625 - 0.1000 g∙mL−1), the temperature (35˚C - 60˚C) and the pH value (2.0 - 6.5) on the total phenolics extracted (mg∙g−1) Y1 and the antiradical activity (%) Y2 as response variables were studied, using RSM [22] . Twenty experiments were performed using a face-centered central composite statistical design for the study of the three independent variables. The levels of the variables were chosen after a series of preliminary experiments.
A second order polynomial model was fitted for the total phenolics extracted and antiradical activity (Y), giving the equation 2 of the following form:
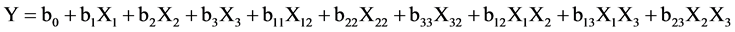 (2)
(2)
where X1, X2, X3 represent the actual levels of the independent variables and b0, bi, bij (i, j = 1, 2, 3) the coefficient estimates, where b0 is the interception, b1 the linear terms, b2 the quadric terms and b3 is the interaction terms.
Statistical analysis was carried out using the MINITABTM statistical software (17.1.0.0). All experiments were run in triplicate and the results expressed as mean ± standard deviation (SD) values. All data were considered statistical significant at p < 0.05.
3. Results and Discussion
3.1. Optimization of the Extraction Conditions
The effect of various solvents such as methanol, ethanol, water and mixtures of them on the production of extracts with the highest total phenolic content was examined. It was found that methanol and ethanol resulted in red and white marc extracts with the maximum values of TPC. Water and chloroform extracts presented lower values of total phenolics content, which is also in agreement with the lower values of antiradical activity (data obtained but not shown). Ethanol was finally selected as the most appropriate solvent, according to Food and Drug Administration, for the extraction of phenolics. Though, in a recent study [23] , the use of methanolic extracts comparing to aqueous or ethanolic extracts has been also reported.
A sample with solid to solvent ratio of 1/30 and 1/5 for grape marc (red and white) and lees (white) respectively, was finally selected. At higher values of this ratio the lower extraction efficiency observed, probably due to mass transfer limitations [2] .
Regarding extraction time, it was found that 2 cycles of 20 min in a sonicator bath were enough for the highest recovery of antioxidant compounds (22.0, 18.0 and 13.0 mg∙g−1 for red, white marc and white lees respectively), whereas 80% of the total phenolics was recovered at the first cycle.
Optimum extraction temperature was estimated at 35˚C (data obtained but not shown). No significant differences were observed at higher temperatures up to 60˚C and this is in agreement with previous reports [2] [24] [25] .
The effect of pH on extraction procedure also tested and it was found that acidic hydrolysis at pH 2.0 using HCl, improves further the recovery of phenolic compounds [26] . The final phenolic content at the conditions resulted in 2.2%, 1.8% and 1.3% for red, white marc and white lees respectively. Interesting, sugars in the same fractions varied from 1.0 for red marc to almost 38% - 43% for the other two samples.
Optimization of solvent extraction using OFAT methodology was followed by RSM. The surface plots obtained by applying RSM for total phenolics content (mg∙g−1) and antiradical activity (%) are shown in Figure 1 and Figure 2 respectively for red marc extract based on the three parameters examined (solid to solvent ratio m/V, pH and temperature). Similar results were obtained with white marc, while differences were noticed with lees where m/V was 1/10 g∙mL−1, and temperature 60˚C.
Table 1 gives the total phenolics content and their antioxidant activity of all wastes extracts examined using RSM and OFAT method, respectively. Small differences observed among the two methodologies can be explained, since OFAT does not include the interactions among the parameters [27] . It is worth mentioning, that RSM values, were close to the predicted responses, verifying the validity of the model.
![]()
Figure 1. Surface plots of total phenolics content (mg∙g−1) of red marc ethanolic extracts, as a function of pH, temperature (˚C) and solid to solvent ratio (g∙mL−1).
![]()
Figure 2. Surface plots of antiradical activity (%) of red marc ethanolic extracts as a function of pH, temperature (˚C) and solid to solvent ratio (g∙mL−1).
![]()
Table 1. Total phenolics versus their antioxidant activity recovered at the optimum conditions determined with RSM and OFAT method. **
* Each value is presented as mean ± SD (n = 3); ** All data were statistical significant at p < 0.05.
3.2. Sorption-Desorption Studies
Various sorbents were tested in order to recover phenolics from initial extracts obtained as described above (Figure 3). Only zeolite and aluminium oxide were found selective towards phenolics, whereas desorption was also feasible. Activated carbon, ash and PVPP sorbed phenolics selectively, but their binding to sorbent surface was irreversible making their desorption negligible.
In an attempt to improve zeolite’s and aluminum oxide’s sorption capacity, pre-treatment with NaOH and CH3OH respectively was performed. As it can be seen from Figure 4, phenolics sorption was enhanced in both
![]()
Figure 3. Effect of various sorbents tested in sorption (■), desorption (□) of phenolics and antioxidant activity ( ) of white marc ethanolic extracts, at w/v 1/30, pH 6.5, contact time 2 h. All data were statistical significant at p < 0.05.
![]()
Figure 4. Effect of sorbent treatment on phenolics sorption after 24 h using non-treated aluminium oxide and zeolite as well as NaOH-treated zeolite and CH3OH-treated aluminium oxide (pH 6.5, sorbent concentration 25 g∙L−1). All data were statistical significant at p < 0.05.
cases and sorbent selectivity towards sugars was also increased. Treatment of zeolite with NaOH aqueous solution leads to negative charge of its surface (by reaction SiOH + OH− = SiO− + H2O) and thus phenolics sorption occurs via hydrogen-bonding between this oxygen site and hydroxyl groups of phenols [28] .
Sorption pH is another important parameter affecting the surface charge of the adsorbents as well as the ionization degree of the sorbate [29] . It was found that in a certain pH range (from 2.0 to 6.5) sorption of most phenolics from all wine extracts was increased from 42% to 68% and from 38% to 93% using NaOH-zeolite and CH3OH-aluminum oxide respectively. At higher pH values (at pH 10.0), formation of a precipitate was observed that was completely dissolved when acidity was reached. This precipitate may be formed due to alteration of chromophoric characteristics under alkaline conditions [30] - [32] .
The sorption percentage of phenolics increased from 10 to 96% by increasing the adsorbent concentration from 5 g∙L−1 to 50 g∙L−1 for white marc extracts. Similar performance was observed with red marc and white lees (data not shown). This may be due to higher number of the available adsorption sites by increasing the sorbent concentration and therefore resulting in higher removal efficiency. Desorption of phenolics followed the same pattern as that of sorption, where at 25 g∙L−1 adsorbent concentration, the maximum phenolic content, possessing high values of the antioxidant activity as well, was recovered with both adsorbents. More specifically, their radical scavenging activity preserved after desorption and ranged between 68% and 85% depending on the type of wine waste used, where red and white marc prevail towards white lees.
Regarding the time profile given in Figure 5, it was found that adsorption of phenolics reached equilibrium within 15 min using CH3OH-treated aluminum oxide. Therefore, considering economic and practical aspects, contact time of 30 min was employed in all subsequent experiments to ensure that equilibrium time was attained. Desorption was also fast, where 15 min needed so as equilibrium to be reached (data obtained but not shown).
Table 2 shows the values of total sugars, phenolics and antioxidant activity of extracts before and after sorption and desorption experiments. Sugars percentage estimated in the desorbed material, was between 5% and 14 % for white lees and marc respectively, whereas in red marc mixtures, sugars had been completely removed, suggesting these two sorbents as appropriate for sorption of phenols. The data in this Table also show that despite the low percentages of the eluted phenolics (0.45%, 0.2% and 0.6% of the extract of red, white marc and white lees respectively), the isolated fractions retain high levels of initial antioxidant activity (69% to 85%). This is very significant finding since desorption elutes only the most of the active phenols, leaving the majority of inactive ones still on the surface of sorbent materials. The desorbed phenolics, as high-added value products may have applicability in food and pharmaceutical industry. The contribution of the major constituents of the obtained fractions, to antioxidant as well as to other biological activities as a result of synergism is under current investigation.
![]()
Figure 5. Effect of time on total phenolics content in the supernatant after sorption with CH3OH-aluminum oxide using red marc (--), white marc (-¡-) at pH 6.5 and white lees (--) at pH 10.0 (adsorbent concentration 25 g L-1). All data were statistical significant at p < 0.05.
![]()
Table 2. Total sugars, phenolics and their antioxidant activity recovered after desorption with 0.1 M HCl using CH3OH- treated aluminium oxide and NaOH-treated zeolite respectively. **
* Each value is presented as mean ± SD (n = 3); ** All data were statistical significant at p < 0.05.
4. Conclusion
Optimized solvent extraction followed by sorption/desorption is proposed here as fast and low cost methodology for the recovery of high-added value phenolics in terms of their radical scavenging activity from wine wastes. The optimum conditions of phenolics extraction, from grape marc and lees, using RSM and OFAT method, were in good agreement. Also, pre-treated, as reported above, of zeolite and aluminum oxide respectively, were selective not only towards phenolic compounds but to sugars as well, based on the studied wine wastes. In conclusion, wine wastes after extraction and subsequent sorption/desorption resulted in the recovery of certain phenolic fractions preserving the 85% of initial antioxidant activity. The results, by applying fast and low cost technique to obtain added value phenolics are of real importance and may have several applications in food and pharmaceutical industry. The contribution of the major constituents of the obtained fractions, to antioxidant as well as to other biological activities as a result of synergism is under current investigation.
Acknowledgements
The authors would like to thank the winery “Ktima Gerovassiliou” for providing wine waste samples of white and red vinification.
The research work was supported by “11SYN_2_1992” action “COOPERATION 2011” of EYDE-ETAK funded by the Operational Program “Competitiveness and Entrepreneurship” (EPAN-II).
NOTES

*Corresponding author.