Fluoride Contamination of Groundwater and Health Hazard in Central India ()
Received 28 October 2015; accepted 7 December 2015; published 10 December 2015

1. Introduction
Abnormal levels of F− in the groundwater are common in India due to weathering of the fractured hard rock pegmatite veins composing of minerals viz. topaz, fluorite fluorapatite, villuamite, cryolite, ferro magnesium silicate, etc. [1] . The F− contamination of groundwater in several states of the country was reported [2] -[21] . Millions of people and animals were exposed to excessive amount of F− through drinking water contaminated from geogenic and anthropogenic sources, suffering with various types of fluoride diseases [22] -[31] . The goal of this work is to study F− contamination of the groundwater and its exposure in domestic animals i.e. cattle and buffalo of Dongargarh city, India.
2. Materials and Methods
2.1. Study Area
Dongargarh (21.18842˚N and 80.75875˚E) is a tourist city in central India with population of 0.1 million inclusive of neighboring villages. The town was settled near majestic mountains. The contaminated groundwater is widely used for drinking, cooking, washing and agricultural purposes. Four minerals i.e. oligoclase, rectorite, kaolinite and feldspar have been identified in the studied area. Feldspar is one of the most dominant mineral constituents of all the above-mentioned rocks, which is highly susceptible to chemical weathering and produces various types of clay minerals [32] .
2.2. Sample Collection
Forty eight groundwater samples were collected from the tube wells of the Dongargarh city from ≈100 km−2 area in the post monsoon (January) and pre monsoon (May) period, 2014 by using established method, Figure 1
![]()
Figure 1. Representation of sampling locations in Dongargarh area, Chhattisgarh, India.
[33] . The groundwater sample was stored in 1-L cleaned polyethylene bottle. The physical parameters i.e. pH, temperature (T), electrical conductivity (EC), dissolved oxygen (DO) and reduction potential (RP) of the water were analyzed at the spot. The water samples were dispatched to the laboratory and preserved in the deep freezer.
The first morning urine sample (100 ml) was collected in plastic bottles containing 0.2 g EDTA. Total 40 urine samples of cattle and buffalo were collected in January, 2014. They were shipped to the laboratory in insulated container at about 4˚C and refrigerated at ?20˚C until use.
2.3. Analysis
The total dissolved solid (TDS) value was determined by evaporation of the filtered water sample (through glass fiber filter) by drying at constant weight. The total hardness (TH) and total alkalinity (TA) values were analyzed by titration methods [34] . The F− content was analyzed by using Metrohm ion meter-781 in the presence of 1:1 total ion strength adjustment buffer (TISAB). The buffer was prepared by adding 58 g NaCl + 5 g CDTA (trans- 1, 2, NNNN, cyclodiamine tetra acetic acid) +57 ml glacial acetic acid and deionized water by adjusting pH value to 5.5 with 8 N NaOH in 1-L volumetric flask.
The ion (i.e. Cl−, 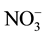 ,
,  ,
,  , Na+, K+, Mg2+ and Ca2+) content of the water was analyzed by Dionex-1100 ion chromatography equipped with the anion and cation columns.
, Na+, K+, Mg2+ and Ca2+) content of the water was analyzed by Dionex-1100 ion chromatography equipped with the anion and cation columns.
The water quality index (WQI) of the groundwater was computed by using the weighed arithmetic method. The value of 6 parameters i.e. pH, DO, EC, TDS, TA and 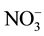 was used in calculation of the WQI with the help of following expression.
was used in calculation of the WQI with the help of following expression.
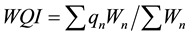
where:
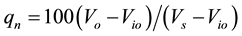
qn = Quality rating of the nth water quality parameter;
Vn = Estimated value of the nth parameter of a given water;
Sn = Standard permissible value of the nth parameter;
Vio = Ideal value of the nth parameter of pure water (i.e., 0 for all other parameters except pH and dissolved oxygen (7.0 and 14.6 mg/L, respectively);
Wn = Unit weight for the nth parameter;
K = Proportionality constant.
Multivariate statistical analysis such as factor analysis (FA) was employed for the source apportionment. The windows statistical software Statistica-7.1 was used for the multivariate statistical calculation.
3. Results and Discussion
3.1. Geological Characteristics of Tube Well
The geological characteristics of the tube wells is summarized in Table 1. The tube wells of the studied area lie in the deeper zone, ranging from 45 - 110 m. The life was ranged from 2 - 50 Yr old. Tube wells are recharged by rain and runoff water during rainy season. The water table is varied from 20 - 50 m, depending on seasons and water uses. The higher T value for deeper tube well was observed due to geothermal energy. In turn, the higher DO value of shallow tube wells was marked.
3.2. Physical Characteristics of Groundwater
The physical characteristics of the groundwater in the post monsoon period is shown in Table 1. The value of pH, DO,T, RP, EC, TDS, TA and TH of groundwater located in 48 tube wells was ranged from 6.0 - 8.1, 8.4 - 9.2 mg/L, 20.0˚C - 25.0˚C, 237 - 330 mV, 221 - 1938 µS/cm, 342 - 2598 mg/L, 128 - 659 mg/L and 99 - 687 mg/L with mean value of 7.2 ± 0.1, 8.9 ± 0.1, 23.0 ± 0.3, 285 ± 5 mV, 861 ± 96 µS/cm, 1138 ± 121 mg/L, 383 ± 47 mg/L and 344 ± 37 mg/L, respectively. Two ions i.e. Na+ and 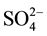 were found to be responsible for contributing the EC value of the water. The DO, EC, TDS, TA and TH value of water was found to be higher than recommended value of 4.0 mg/L, 300 µS/cm, 500 mg/L, 120 mg/L and 300 mg/L, respectively [35] [36] . The
were found to be responsible for contributing the EC value of the water. The DO, EC, TDS, TA and TH value of water was found to be higher than recommended value of 4.0 mg/L, 300 µS/cm, 500 mg/L, 120 mg/L and 300 mg/L, respectively [35] [36] . The
![]()
Table 1. Characteristics of tube well and groundwater in the post monsoon period, 2014.
RP value of water was marked to be just of a half of recommended value of 600 mV.
3.3. Chemical Characteristics of Groundwater
The chemical characteristics of the groundwater in the post monsoon period is shown in Table 2. The concentration of ions i.e. F−, Cl−, 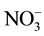 ,
, 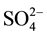 ,
, 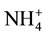 , Na+, K+, Mg2+ and Ca2+ in the groundwater of 48 tube wells was ranged from 2.0 - 10.3, 43 - 408, 48 - 152, 12 - 161, 10 - 144, 8.0 - 75, 3.0 - 25, 9.0 - 57 and 24 - 172 mg/L with mean value of 4.9 ± 0.5, 121 ± 22, 75 ± 8, 47 ± 9, 55 ± 11, 41 ± 5, 6.1 ± 1.5, 28 ± 3 and 87 ± 9 mg/L, respectively. The F− and
, Na+, K+, Mg2+ and Ca2+ in the groundwater of 48 tube wells was ranged from 2.0 - 10.3, 43 - 408, 48 - 152, 12 - 161, 10 - 144, 8.0 - 75, 3.0 - 25, 9.0 - 57 and 24 - 172 mg/L with mean value of 4.9 ± 0.5, 121 ± 22, 75 ± 8, 47 ± 9, 55 ± 11, 41 ± 5, 6.1 ± 1.5, 28 ± 3 and 87 ± 9 mg/L, respectively. The F− and 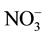 crossed the recommended limit of 1.5 and 45 mg/L, respectively in the water of all tube wells [35] [36] . However, Mg and Ca concentration was above recommended limit of 30 and 75 mg/L in the 33% and 67% tube wells [35] [36] .
crossed the recommended limit of 1.5 and 45 mg/L, respectively in the water of all tube wells [35] [36] . However, Mg and Ca concentration was above recommended limit of 30 and 75 mg/L in the 33% and 67% tube wells [35] [36] .
3.4. Seasonal Variation and Sources of Fluoride
The chemical data for the pre monsoon period, 2014 is presented in Table 3. The variation of physical and chemical parameters of the water in the pre monsoon period (May 2014) is presented in Figure 2. The value of pH, EC, TDS, TA, TH, F, Na, Mg and Ca was found to be increased ≥30%, may be due to increase of water temperature, ≈4˚C, and deduction of water level up to 50 m. The F− with the metals i.e. Na+, Mg2+ and Ca2+ had good correlation (r = 0.78 - 0.85), indicating origin from the rock weathering, Figure 3. Other ions (i.e. Na+, Mg2+, Ca2+, Cl− and ) among themselves had fare correlation, suggesting origin from multiple sources, Table 4. However, two ions i.e.
) among themselves had fare correlation, suggesting origin from multiple sources, Table 4. However, two ions i.e. 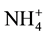 and
and ![]() had good correlation, originating from similar anthropogenic sources.
had good correlation, originating from similar anthropogenic sources.
3.5. Factor Analysis
The factor analysis of data has extracted six factors which explained 77.25% of the variance in the data set. The loadings of variables, eigenvalues and cumulative variance for each factor are shown in Table 5. Factor-1 accounted for 32.66% of the total variance with high positive loadings of Ca2+, Mg2+, F− and TH. This factor suggests the role of dissolution/precipitation processes of some minerals such as CaF2 and CaCO3. Factor-2 accounted for 13.31% of representation with strong positive loading of pH which is negatively correlated with redox potential (RP). This factor suggests occurrence of redox processes which determine the acidic or alkaline nature of groundwater. Factor-3 represents 10.80% of the total variance with strong positive loadings of ![]() and
and![]() . This factor loadings shows the anthropogenic influences on these parameters. Factor-4 yielded 8.39% of the total variance with strong positive loadings of
. This factor loadings shows the anthropogenic influences on these parameters. Factor-4 yielded 8.39% of the total variance with strong positive loadings of ![]() and Na+ suggesting mineral weathering. Factor-5 accounted for 6.32% of the total variance with high positive loading of K+. In groundwater, K could proceed from fertilizers or weathering of K-feldspar. Factor-6 accounted for 5.66% of the total variance with a high positive value of temperature (T). The T value is a variable which controls many reactions.
and Na+ suggesting mineral weathering. Factor-5 accounted for 6.32% of the total variance with high positive loading of K+. In groundwater, K could proceed from fertilizers or weathering of K-feldspar. Factor-6 accounted for 5.66% of the total variance with a high positive value of temperature (T). The T value is a variable which controls many reactions.
3.6. Water Quality Index
The WQI of the water in the post monsoon period was ranged from 22 - 226 with mean value of 97 ± 12. The value of TDS, TA, TH, F− and ![]() in the water of all tube wells was found above permissible limits of 500, 120, 300, 1.5 and 45 mg/L, respectively [35] [36] . However, in the pre monsoon period, the value of F−, Mg2+ and Ca2+ crossed significantly the prescribed permissible limit of 1.5, 30 and 75, making water unsafe for drinking purposes.
in the water of all tube wells was found above permissible limits of 500, 120, 300, 1.5 and 45 mg/L, respectively [35] [36] . However, in the pre monsoon period, the value of F−, Mg2+ and Ca2+ crossed significantly the prescribed permissible limit of 1.5, 30 and 75, making water unsafe for drinking purposes.
3.7. Fluoride Toxicities
Chronic ingestion of fluoride water in endemic areas leads to development of fluorosis in the animal e.g. dental discoloration, difficulty in mastication, bony lesions, lameness, disability and mortality [37] . In lower age group, the lesion of teeth, skin, hair and nails were frequently observed. Fluoride enters the animal body mainly through the intake of water and quickly absorbed in the gastrointestinal tract. The excess F− is excreted largely through the urine. The survey for the fluorosis in domestic animals (3 - 15 Yr) in the Dongargarh area, Rajnandgaon, Chhattisgarh, India was carried out in January 2014. A total of 40 domestic animals were screened for prevalence of various types of fluorosis i.e. lesion, dental, horn skin and toe fluorosis. The F− concentration
![]()
Table 2. Chemical characteristics of groundwater in post monsoon period, 2014, mg/L.
![]()
Table 3. Characteristics of groundwater in pre monsoon period, May 2014.
![]()
Table 4. Correlation matrix of ions.
![]()
Table 5. Eigenvalues and factor loadings of Dongargarh groundwater samples.
Significant factor loading in bold >0.7.
in their urine samples were measured and presented in Table 6. The concentration of F− in the buffalo and cattle urines was ranged from 18 - 52 and 26 - 58 mg/L with mean value of 31 ± 4 and 41 ± 4 mg/L, respectively. At least 7 - 10 folds higher F− content in the urine of animals was marked, may be due intake of higher dose of F-contaminated water and food. The higher fluorosis prevalence rate was observed in the cattle than buffalo, Figures 4-6.
![]()
Table 6. Fluoride exposure in animals.
DF = Dental fluorosis, SF = Skin fluorosis, TF = Toe fluorosis, HF = Horn fluorosis, L = Lesion.
![]()
Figure 2. Seasonal variation of ionic concentration in post (a) and pre (b) monsoon period, 2014.
![]()
Figure 3. Correlation of F− with Na+ (a), Mg2+ (b) and Ca2+ (c).
4. Conclusion
The groundwater of Dongargarh is contaminated with F− at dangerous levels due to mineralization of the bed rock F− in the water. The WQI index of water was found to be ≈100, making water unsafe for dinking purposes. The F− levels in urine of cattle and buffalo were found several folds higher than recommended value of 4 mg/L. Around 5% domestic animals of the studied were suffered with different types of fluorosis.
Acknowledgements
We are thankful to the UGC, New Delhi for awarding Rajiv Gandhi Fellowship to one of the author: NSD.
NOTES
![]()
*Corresponding author.