Cr(VI) Removal from Aqueous Solution by Chitosan/Carboxylmethyl Cellulose/Silica Hybrid Membrane ()
1. Introduction
Chitosan (CS), N-deacetylated form of chitin, is the second abundant renewable biopolymer, next to cellulose [1] [2]. Due to its many unique properties such as good coagulating properties, excellent forming film characteristics, nontoxic, environment-friendly, low-cost and high-efficiency, high contents of amino and hydroxyl functional groups in the chains, recently, it has attracted great attention as a new biosorbent for the significant adsorption potential for the removal of heavy metal ions from textile effluents [3] [4]. However, chitosan membrane is highly swollen in water, which may change its physical structures. On the other hand, the low mechanical strength and poor acid resistance of chitosan membrane are also to be overcome. Blending chitosan with effective compatible polymers, such as carboxymethyl cellulose (CMC), acrylonitrile butadiene styrene and polyvinyl alcohol, etc, is an effective way to strengthen its structure
[5]
. Owing to its opposite electric charge, CMC can strongly react with CS and acts as an ionic cross-linking agent at the appropriate pH9 [6]-[8]. The composite of CMC/CS has been investigated by some researchers for possible medical applications because of its excellent biocompatibility, biodegradability and hydrophilic [8]-[10]. Some literatures also reported the fabrication of porous CMC/CS membranes and application for pervaporation (PV) dehydration and adsorption metal ion [11] [12].
In this work, chitosan and carboxymethyl cellulose were provided as the substrate, using 3-glycidoxypropy- ltrimethoxysilane (GPTMS) as crosslinking agent. Chitosan/carboxymethyl cellulose/silica (CS/CMC/silica) hybrid membranes were prepared and used to remove Cr(VI) ions in effluent. The hybrid membranes were characterized by FTIR, SEM. Batch adsorption experiments were carried out to investigate the effects of different conditions, such as the adsorbent dosage, Cr(VI) ions concentration, adsorption temperature, and solution pH and contact time, on the adsorption capacity of hybrid membrane for Cr(VI) ions. The adsorption behavior of the hybrid membranes were analyzed by the pseudo-first-order kinetic model and pseudo-second-order kinetic model.
2. Experimental
2.1. Materials and Characteration
Chitosan (CS, degree of deacetylation 96.31%, MW = 7.9 × 105 Da) was purchased from Zhejiang Jinke Bio- tech Co. Ltd. (Zhejiang, China). Carboxymethyl cellulose sodium salt (CMC, M.W. = 800), K2Cr2O7 (analytical pure) were purchased from Guoyao Chemical Co. Ltd. (Shanghai, China). 3-Glycidoxypropyltrimethoxysilane (GPTMS) was supplied by Yancheng Renbo Chemical Company (Yancheng, China).
FTIR spectra of the hybrid membrane were recorded using NEXUS-670 (Nicolet, USA) over the range 500 - 4000 cm−1. The samples were ground to a very fine powder and mixed with a highly dried KBr powder (200 mg), then pressed to transparent tablet. SEM images of the hybrid membrane were taken using a scanning electron microscope FEI Quanta 200 scanning Electron Microscope (USA). The samples were sputtered with gold, and operated at 15 kV.
2.2. Membrane Preparation
To obtain CS/CMC/silica hybrid membranes, chitosan solution 2% (w/v) and CMC aqueous solutions 2% (w/ v) were first prepared by dissolving chitosan and CMC in acetic acid 1% (v/v) and distilled water, respectively. Then, chitosan solution 2% was added into CMC solution 2% until VCMC:VCS = 1:1 with stirring. 1% (v/v) GPTMS were drop-wise added into the CS and CMC mixtures, the mixed solutions were adjusted to pH 1 - 2 using HCl solution and stirred for 4 h until an obviously pale yellow viscous liquid occurred. The obtained pale yellow viscous liquid was cast into a clean smooth slide to form membrane in an oven (50˚C) for 24 h. The membrane were neutralized in NaOH solution (
0.1 M
) for about 10 min, washed thoroughly with distilled water, and dried again. Finally, CS/CMC/silica hybrid membranes were obtained and named as CSMH.
2.3. Adsorption Experiments
Batch experiments were conducted by placing hybrid membranes in 250 mL bottle containing Cr(VI) ions. The effect of temperature (30˚C - 70˚C) on the adsorption was evaluated at pH 7.0. The effect of the initial concentration of Cr(VI) ion on the adsorption was carried out in the solution (20 mg/L - 200 mg/L), at 30˚C and pH 7.0. The effect of initial pH value 1 - 9 (adjusted with
0.2 M
HCl or
0.2 M
NaOH) was conducted in Cr(VI) ion solution. The bottles were agitated for 120 min. The Cr(VI) ion concentrations in the filtrate after filtration and the initial concentrations were determined by UV-vis spectrometer. The adsorption capacities were calculated by Equation (1).
 (1)
(1)
where qe is the adsorption capacities of hybrid membrane (Cr(VI) ion/g adsorbent), V is the volume of Cr(VI) ion solution (L), C0 is the initial concentration of Cr(VI) ion before adsorption (mg/L), Ce is the concentration of Cr(VI) ion after adsorption (mg/L), that is in filtrate, and m is the weight of hybrid membrane (g).
3. Results and Discussion
3.1. FT-IR
The FT-IR spectra of chitosan, CMC and CSMH hybrid membrane are shown in Figure 1. CS presented a
![]()
Figure 1.FT-IR spectra of chitosan, CMC and hybrid membrane.
characteristic band at 3450 cm−1 which was attributed to -NH2 and -OH stretching vibration and 1652 cm−1 which was characteristic of amide group
[13]
. The FTIR spectrum of CMC showed carboxylate ion -COO- antisymmetric stretching peaks at 1600 cm−1, symmetrical C-O stretching at 1059 cm−1, and C-O-C group at 1630 cm−1
[14]
. The FTIR spectrum of hybrid membrane demonstrated a broad band at 3400 cm−1 corresponding to N-H stretching (amide II and primary amine of hybrid membrane) and -OH stretching. The bands at around 2933 cm−1 can be assigned to the C-H stretching (methyl group) of hybrid membrane, and 1092 cm−1 and 1200 cm−1 (C-O-C, polysaccharide ring). The hybrid membrane had 1596 cm−1 (N-H) and 1740 cm−1 (C=O) which verified that the composite was consist of CS and CMC. The band at 1630 cm−1 became sharper due to the formation of intermolecular interactions between CMC and CS
[15]
. The peak of asymmetry stretching of COO- was also found at 1420 cm−1. It showed that the strong electrostatic attraction between [ ] of CS and [COO−] of CMC may be the main ion cross-linking interaction leading to the formation of polyelectrolyte network structure. At the same time, -OH has slight band-shifts in composite scaffolds, which means that there are some inter- or intra-hydrogen bonds among these three components of CS/CMC/silica composite membrane. This is agreement with the results from Jang
[ 11 ]
. The vibration frequencies of NH, Si-O-Si, Si-O-H and Si-O were at 1422 cm −1, 1092 cm −1, 914 cm −1 and 762 cm −1, respectively, which indicated the possible cross-linking between CMC, CS and GPTMS.
] of CS and [COO−] of CMC may be the main ion cross-linking interaction leading to the formation of polyelectrolyte network structure. At the same time, -OH has slight band-shifts in composite scaffolds, which means that there are some inter- or intra-hydrogen bonds among these three components of CS/CMC/silica composite membrane. This is agreement with the results from Jang
[ 11 ]
. The vibration frequencies of NH, Si-O-Si, Si-O-H and Si-O were at 1422 cm −1, 1092 cm −1, 914 cm −1 and 762 cm −1, respectively, which indicated the possible cross-linking between CMC, CS and GPTMS.
3.2. Morphology of Hybrid Membrane
The surface morphology of the hybrid membrane was taken using SEM and shown in Figure 2. From Figure 2(a), it can be seen that the pure chitosan membrane has relatively smooth and plain surface. Incorporating silane couple agent into chitosan/carboxymethyl cellulose membrane can affect the surface morphology of the hybrid membrane significantly. As seen from Figure 2(b), uneven membrane surface has been formed, “like island”small protuberant are basically covered on surface of CSMH hybrid membrane and they closely adjacent. The smaller size pores are also observed for CSMH. Because of strong electrostatic interaction between chitosan and sodium carboxymethylcellulose, crosslinking reaction with silane coupling agent occurred at hydroxyl position. Therefore, porous structure in hybrid membrane can be attributed to forming -O- connection between the silicon coupling agent and hydroxyl sites on chitosan and sodium carboxymethyl cellulose [12] [13].
3.3. Effect of Different Factors on Adsorption Properties of the Hybrid Membrane
To investigate the effect of the adsorbent quantity on the adsorption capacities, the
160 g
/mL Cr(VI) solutions with pH 7.0 were stirred for 2 h with different amount of CSMH hybrid membrane from 0.1 to
0.5 g
. The result is shown in Figure 3(a). The adsorbed quantity of Cr(VI) per unit mass of the adsorbent increased slowly with the increase of adsorbent up to
0.3 g
, and after the critical dose, the adsorption capacities decreased. This increase could be attributed to the increase of the adsorbent surface area and availability of more active
![]()
Figure 2. Morphology of pure chitosan and hybrid membrane: (a) Pure chitosan membrane; (b) CSMH.
adsorption sites on the hybrid membrane surface with the increase of it. But when adsorbent dose reached
0.3 g
, the adsorption capacity of the hybrid membrane decreased for Cr(VI). Therefore, the hybrid membranes of
0.3 g
were used for Cr(VI) in the following experiment. The effect of initial Cr(VI) concentration on the adsorption efficiency of the hybrid membrane was investigated (shown in Figure 3(b)). With the Cr(VI) concentration increasing, the adsorption capacity per unit mass of the hybrid membrane increased. The higher initial concentration of Cr(VI) provided an important driving force to overcome the mass transfer resistance for Cr(VI) transfer between the solution and the surface of the hybrid membrane. In the process, the Cr(VI) primarily overcame the boundary layer effect and then diffused from surface to inside of the hybrid membrane.
Temperature is an important controlling factor in the practical applications of the proposed adsorbing process since the most of the textile effluents is produced at relatively high temperatures. The results (shown in Figure 3(c)) indicated the adsorption capacity of Cr(VI) onto the hybrid membrane decreased with an increase in temperature and it indicated that the process was exothermic. The adsorption capacities of Cr(VI) on the hybrid membrane were examined in different pH solution at initial dye concentration 120 mg/L, contacting time 2 h by the batch experiments. The results are shown in Figure 3(c). Cr(VI) adsorption followed a typical metal anion adsorption behavior, where maximum adsorption took place at low pH between 1 and 2. With the increase of pH, the adsorption capacities decreased. It was attributed to the deprotonation of the hybrid membrane, which reduced the chelation and absorption of Cr(VI). High adsorption at acidic pH conditions could be explained by the chemical character of the chromium species and the adsorbent surface. In the acidic pH range (1 - 2), the prominent formation of chromium are 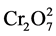 -,
-, 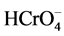 , and above pH 7.0 the primary stable species is
, and above pH 7.0 the primary stable species is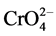 . Therefore, at lower pH,
. Therefore, at lower pH,  - and
- and 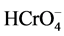 were adsorbed onto the hybrid membrane, resulting in a high percentage of adsorption. At basic pH condition, the suppression of the hydrolysis of Cr(VI) may be the reason for the decreased adsorption.
were adsorbed onto the hybrid membrane, resulting in a high percentage of adsorption. At basic pH condition, the suppression of the hydrolysis of Cr(VI) may be the reason for the decreased adsorption.
3.4. Adsorption Kinetics
The adsorption kinetics of Cr(VI) onto the hybrid membrane was investigated under the condition of the 120 mg/L Cr(VI) at neutral. The samples were taken out at different time intervals in the range of 0 - 240 min. The results are shown in Figure 4. It can be seen that the adsorption capacity for Cr(VI) increased with the increase of contacting time. The Cr(VI) reached equilibrium at 60 min, The Cr(VI) uptake potential indicate that most of the active sites of the hybrid membrane were exposed for interaction with the Cr(VI) ion. In order to investigate the mechanism of the adsorption process, the pseudo-first-order equation, the pseudo-second-order equation and particle diffusion equation based on adsorption equilibrium capacity can be expressed by Equations (2)-(4) [14] [15] respectively.
 (2)
(2)
 (3)
(3)
 (4)
(4)
where qe (mg/g) and qt (mg/g) are the adsorption quantity at adsorption equilibrium and the adsorption quantity at time t (min), respectively, k1 (min−1) and k2 (min∙g∙mg−1) are the kinetic rate constants for the pseudo-first- order equation and the pseudo-second-order equation, respectively. The slopes and intercepts of plots of l − ln [(qe − qt)/qe] versus t are used to determine the pseudo-first-order rate constant k1 and qe. The slopes and intercepts of plots of t/qt versus t are used to calculate the pseudo-second-order rate constant k2 and qe. ki and C are respectively rate constant and constant for the particle diffusion equation [16] [17]. The results are shown in Figure 4(b). The calculated results are summarized in Table 1. It is found that the adsorption behavior of free
![]() (a) (b)
(a) (b)
Figure 4. (a) Adsorption kinetics curves of hybrid membrane; (b) Test of pseudo first-order and second- order equation for Cr(VI).
![]()
Table 1. Comparison of the pseudo-first and pseudo-second order constant.
a (at 30˚C,C0 = 120 mg/L, pH neutral).
Cr(VI) ions could be best described by the pseudo-second-order model. This result indicates that chemisorption mechanism might play an important role for the adsorption of Cr(VI) ions onto the hybrid membrane.
4. Conclusion
By using glycidoxypropyltrimethoxysilane (GPTMS) as crosslinking agents, chitosan/carboxy methylcellulose/silica hybrid membrane was prepared. The hybrid membranes contained COOH, NH3+ and -O- functional groups. The hybrid membrane could be employed as an adsorbent for Cr(VI) removal from waste effluents in acid medium. Adsorption equilibrium of hybrid membrane on Cr(VI) ion was reached in 60 min. The kinetic data conformed better to the pseudo-second order equation. Chemisorption mechanism might play an important role for the adsorption of Cr(VI) ions onto the hybrid membrane.
Acknowledgements
The project was supported by the agricultural science and technology innovation projects of Yancheng (No.YKN2014014).