Synthesis and Crystal Structure of the First Example of a Tris-Chelated Co(II) Complex Based on Oxamide Dioxime Ligand ()
1. Introduction
Transition metal complex cations of the form [M(H2oxado)3]n+ (M = metal(II) or metal(III), n = 2 or 3, H2oxado = oxamide dioxime), constitute an interesting family of chiral and―eventually―paramagnetic cations, due to their molecular structure (geometry and volume) that predestines them to undergo straightforward chemical combinations with suitable anionic counterparts such as the oxalatometalate series, [M’(C2O4)3]3− (M’ = metal(III)), thus generating a wide range of multifunctional crystalline materials [1] required in the development of emerging technologies [2] -[5] . It has been observed previously that in aqueous solution, the CoII ion generally reacts with the oxamide dioxime ligand, yielding the familiar trischelated CoIII complex cation, [Co(H2oxado)3]3+ [6] -[8] . In our current research program which is focused on fabricating multifunctional materials involving N,N’- or O,O’- chelating ligands, the [Co(H2oxado)3]3+ building block and its homologous nickel(II) complex, [Ni(H2oxado)3]2+, have proven to be good partners in the formation of hydrogen-bonded functional solid-state assemblies [9] [10] .
Along the line of this research program, an attempt to prepare the elusive tris(oxamide dioxime)cobalt(III) tris(oxalato)cobaltate(III), [Co(H2oxado)3][Co(C2O4)3]・nH2O, similar to [Co(H2oxado)3][Cr(C2O4)3]・5H2O [9] led to the serendipitous isolation of the cobalt(II) title compound, [Co(H2oxado)3]C2O4・H2oxado・2H2O, the structure of which is reported herein. The present finding turns out to be quite interesting, in as much as it provides, to the best of our knowledge, the first example of a complex salt containing the dipositive [Co(H2oxado)3]2+ ca- tion, as highlighted in Scheme 1.
2. Experimental
2.1. Materials and Measurements
The organic ligand H2oxado (analytical grade) was freshly prepared by the condensation of dithiooxamide (98.5%, Fluka) and hydroxylammonium chloride (99%, Merck) in presence of sodium carbonate (99.5%, Prolabo) as previously reported [9] [11] . The tripotassium tris(oxalato)cobaltate(III) trihydrate salt, K3[Co(C2O4)3]・3H2O, was synthesized as described by Bailar & Jones [12] . All other chemicals were purchased and used as received. Elemental analysis was performed on a VARIO EL (Heraeus) CHNS analyzer.
2.2. Synthesis of [Co(H2oxado)3]C2O4・H2oxado・2H2O
The title compound was obtained as follows: dark green crystals of K3[Co(C2O4)3]・3H2O (0.5 g, 1 mmol) were dissolved in H2O (50 mL) acidified with a drop of HNO3. To the filtered solution were added with stirring at 30˚C successive small portions of oxamide dioxime (0.36 g, 3 mmol), followed by portions of finely powdered Co(NO3)2・6H2O (0.3 g, 1 mmol). The reaction mixture was stirred magnetically for 1 h and allowed to decant over 2 h. The reddish-pink precipitate of CoC2O4 was carefully filtered off, and the solution left to evaporate slowly in a hood at room temperature. After two weeks, prismatic reddish crystals suitable for single crystal X-ray studies were harvested. Anal. Calcd. for C10H28CoN16O14: C, 18.33; H, 4.30; N, 34.20%. Found: C, 18.32; H, 4.30; N, 34.18%.
2.3. Crystal Structure Determination and Refinement
A suitable single crystal of the title compound with dimensions 0.5 × 0.12 × 0.10 mm was mounted on a glass
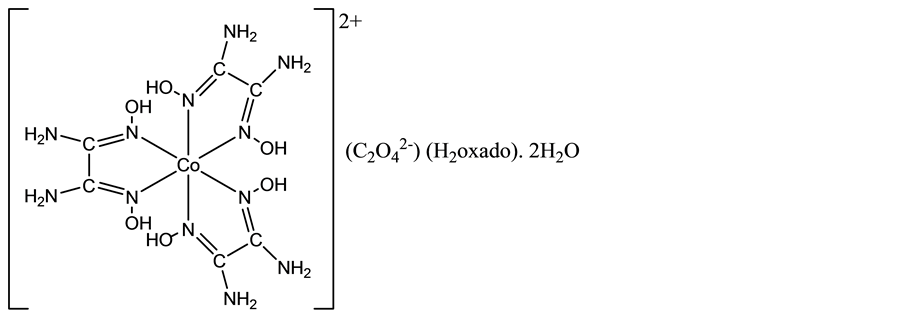
Scheme 1. Chemical diagram of the title compound.
fiber and fixed to the goniometer head. Data collection [13] was carried out on a Bruker-Nonius X8 kappa APEX II CCD area-detector diffractometer using graphite-monochromatic radiation λ(Mo Kα) = 0.71073 Å at 296(2) K. Data reduction was performed using SAINT [13] , and absorption corrections were carried out by multi-scan method by SADABS [14] . The structure was solved by direct methods and refined against F2 by full-matrix least-squares techniques with SHELXTL [15] . All non-hydrogen atoms were refined with anisotropic displacement parameters. The hydrogen atoms were included from calculated positions and refined riding on their respective parent atoms with isotropic displacement parameters. Crystal data and structure refinement de- tails for the title compound are summarized in Table 1, and selected bond lengths and angles in Table 2.
3. Results and Discussion
Single-crystal X-ray structural analysis reveals that the title compound crystallizes in the triclinic space group P-1. It is formulated [Co(H2oxado)3]C2O4・H2oxado・2H2O. As shown in Figure 1, the molecular structure is composed of one [Co(H2oxado)3]2+ complex cation, one oxalate (2-) anion, one oxamide dioxime molecule and two solvent water molecules. The point of prime interest in this report is the assessment of the existence of the pseudo-octahedral complex cation, tris(oxamide dioxime)cobalt(II), [Co(H2oxado)3]2+, and this, as far as we know, is raised here for the first time. The oxamide dioxime acting as a crystallization molecule has been described previously [16] . Selected bond lengths and angles of the title compound are listed in Table 2, and compared with the corresponding values (in brackets) for the reported [Co(H2oxado)3]3+ cation [6] -[8] . Data derived from this tripostive cation reflect nicely the C3 symmetry of the complex entity, whereas those derived from the dipo- sitive complex cation, [Co(H2oxado)3]2+, deviate noticeably from this symmetry. However, the pseudo-octahedral coordination of the central cobalt and the chiral nature of the complex cation are maintained. Whereas the O?N bond lengths within the [Co(H2oxado)3]2+ entities of the present structure range from 1.290(3) to 1.490(2) Å, its average value (1.383 Å) is slightly shorter than O?N bonds in the familiar [Co(H2oxado)3]3+ cation whose common value is 1.391(6) Å (Table 2). The projection of the structure along [100] highlights the [Co(H2oxado)3]2+ ions disposed in centrosymmetric dimers leading to a zig-zag chain running along c axis (Figure 2). The three- dimensional crystal packing of the title compound is reinforced by extended O?H∙∙∙O, O?H∙∙∙N and N?H∙∙∙O bridges which interlink the complex cations, [Co(H2oxado)3]2+, oxalate anions, oxamide dioxime molecules and water molecules.
![]()
Figure 1. An ORTEP drawing of [Co(H2oxado)3]C2O4・H2oxado・2H2O show- ing atom-labelling scheme with displacement ellipsoids drawn at 50% probability level.
![]()
Table 1. Crystal data and structure refinement details for the title compound.
![]()
Figure 2. Projection of the achiral unit cell of the title compound along [100], showing centro-symmetric dimerization of CoII complex cations and interconnection of these dimers, C2O42− dianions, H2oxado and H2O molecules via hydrogen bridges (dotted lines).
![]()
Table 2. Selected bond lengths (Å) and angles (˚) for the title compound*.
*Comparative values in square brackets are from ref. [7] .
*Corresponding author.
4. Conclusion
We have isolated from aqueous solution the salt [Co(H2oxado)3]C2O4・H2oxado・2H2O containing the unprece- dented tris-chelated CoII complex cation, [Co(H2oxado)3]2+. In this cation, as well as in the familiar [Co(H2oxado)3]3+ ion, the central cobalt atom is in the same pseudo-octahedral coordination geometry of the six imino N atoms of H2oxado. In the crystal, the [Co(H2oxado)3]2+ ions are hydrogen-bonded into centrosymmetric dimers, thus gen- erating zig-zag chains running parallel to [001]. It is interesting to note that the dipositive complex cation [Co(H2oxado)3]2+ appears as resulting from the reduction of the tripositive complex cation [Co(H2oxado)3]3+, a process reminiscent of catalytic or biological reactions involving transfer of electrons.
Acknowledgements
We thank Professor You Song (Nanjing University, China) and his research team for their help in the X-ray structural analysis.
Appendix A. Supplementary Material
Detailed crystallographic data in CIF format for this paper were deposited with the Cambridge Crystallographic Data Centre (CCDC-839359). The data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html [or from Cambridge Crystallographic Data Centre (CCDC), 12 Union Road, Cambridge CB2 IEZ, UK; fax: +44 (0) 1223-336033; e-mail: deposit@ccdc.cam.ac.uk].
NOTES
*Corresponding author.