Physical and Electrolytic Properties of Monofluorinated Ethyl Acetates and Their Application to Lithium Secondary Batteries ()
1. Introduction
Ethyl acetate is a carboxylate ester of ethanol and acetic acid and is commonly abbreviated to EA or EtOAc. This colorless liquid has a characteristic sweet smell (similar to pear drops). EA is used as a solvent for chemical reactions. EA is often used in cosmetics because of its odor, and its smell is associated with nail polishes. Furthermore, EA is used in confectionery, perfumes, and fruits because it evaporates at a fast rate, leaving but the scent of the perfume on the skin.
EA is one of the linear carboxylates. EA shows high relative permittivity (εr = 6.02 at 25˚C), but low viscosity (η = 0.426 mPa s at 25˚C) [1] , as compared to dimethyl carbonate (DMC) (εr = 3.12 and η = 0.63 mPa s at 25˚C), ethyl methyl carbonate (EMC) (εr = 2.93 and η = 0.68 mPa s at 25˚C), and diethyl carbonate (DEC) (εr = 2.82 and η = 0.75 mPa s at 25˚C). These three linear carbonates are commonly used as low-viscosity solvents for lithium-ion batteries [2] [3] . In contrast, ethylene carbonate (EC) is a cyclic carbonate and is used as a high-po- larity solvent for lithium-ion batteries [2] [3] .
The use of linear carboxylates as alternative solvents can decrease the internal resistance of lithium batteries. However, the potentials for the oxidation decomposition of linear carboxylates (3.4 V vs. SCE for methyl acetate and methyl propionate [4] ) are lower than those of linear carbonates (3.7 V vs. SCE for DMC, EMC, and DEC [4] ). Partially fluorinated carbonates exert the polar effect on the physical and electrochemical properties such as relative permittivity, viscosity, electrolytic conductivity, and electrochemical stability [5] -[11] . Monofluorination of EA and ethyl propionate (EP) improved the anodic stability [12] [13] . Methyl difluoroacetate is known as an excellent additive for lithium batteries [2] [14] -[18] .
2-Fluoroethyl acetate (2FEA) and ethyl fluoroacetate (EFA) are isomeric with each other: structural isomerism, which is especially called position isomerism. Figure 1 compares the structures of 2FEA, EFA, EA, and EMC. In this paper, we describe the temperature dependence of the mass densities, molar concentrations, relative permittivities, and viscosities of 2FEA, EFA, EA, and EMC in some detail. Furthermore, we report the conductivities of electrolyte solutions in 2FEA, EFA, EA, and EMC and their application to lithium secondary batteries.
2. Experimental
The synthesis and purification of 2FEA are described in the previous paper [12] . The apparatus and techniques for measurements are essentially the same as those previously reported [6] [10] .
Relative permittivities (εr) or relative dielectric constants were measured with a LF impedance analyzer (Hewlett Packard, 4192A) connected to a thermostat (Ando Denki, TO-9). The electrostatic capacitances of air (C0) and a sample (Csample) were measured at a frequency of 1 MHz, and the relative permittivity can be adequately approximated by the ratio (εr ≈ Csample/C0). The samples were sufficiently deaerated by bubbling Ar gas (99.9%) before the measurement.
Kinematic viscosities (ν) were measured with a capillary-tube viscometer, Ostwald viscometer (Shibayama Scientific Co., Ltd., SS-290S), equipped with a thermostat. Silicone oil was circulated in the constant-tempera- ture bath. Viscosity (η) or viscosity coefficient can be expressed as the product of the kinematic viscosity and the mass density (η = ν・d); the kinematic viscosity is defined as the ratio of the viscosity to the mass density of a fluid (ν = η/d) and is directly proportional to the time required for the liquid to flow through a capillary-tube viscometer under its own hydrostatic head. Mass densities (d) were measured by the use of a density/specific gravity meter (Kyoto Electronics Manufacturing Co., Ltd., DA-505). The physical constants of organic solvents were measured from 10˚C to 70˚C.
Conductivities of electrolyte solutions were measured by use of a conductometer (Toa Electronics Inc., Model CM-60S) equipped with the cell (Model CGT-511B) from −5˚C to 70˚C.
Linear potential sweep voltammetry (LSV) was performed with a platinum electrode, 1.6 mm in diameter, on a computer-controlled electrochemical system (Solartron Analytical, type SI1287) at a sweep rate of 5 mVs−1.
![]()
Figure 1. Structures of 2FEA, EFA, EA, and EMC.
Lithium foil and a platinum wire were used as the reference and the auxiliary electrodes, respectively.
Coulometric efficiency achieved with the repetitiveness of the deposition and dissolution of lithium on a nickel electrode (cycling efficiency of a lithium anode) was measured with a charge/discharge unit (Hokuto Denko Corp., Model HJ-201B). Three-electrode cells were used for the measurement of the cycling efficiency. Lithium foil was employed as the auxiliary and the reference electrodes. The cycling efficiency was estimated by a galvanostatic plating/stripping method reported by Koch and Brummer [19] . The plating and the stripping current densities were set at ±1 mAcm−2. The plated charge density was adjusted to −300 mCcm−2, and the cut- off voltage was set at +1.0 V vs. Li|Li+ during the anodic stripping. A 2025-type coin cell (can size: 20 mm in diameter and 2.5 mm in thickness, stainless steel body) was assembled with a LiCoO2 sheet cathode (16 mm in diameter), a lithium-metal sheet anode (16 mm in diameter), a separator (Celgard Inc., #3501), and the test solution. The discharge capacities of the Li|LiCoO2 coin cells were measured with a 6-channel charge/discharge unit (Hokuto Denko Corp., Model HJ-101SM6). The coin cells were charged in a constant current (1 C)-constant voltage (4.2 V) regime at 25˚C until total charge time reached 1 h. Afterwards, they were discharged to 3.0 V at the inverse current of 1 C.
The preparation of electrolyte solutions and the fabrication of three-electrode cells and coin cells were carried out in an argon-filled glove box system made by VAC. The concentrations of O2 and H2O were kept less than 1 ppm in the glove box.
The morphology of the films formed on the Ni (working) electrode after cycling was observed by using a scanning electron microscope (SEM, JSM-5310).
3. Results and Discussion
3.1. Physical Properties of Solvents
Figure 2 shows the temperature (θ) dependence of (a) mass densities (d) and (b) molar concentrations (c) of 2FEA, EFA, and EA single solvents. In addition, this figure compares data for EMC, whose structure is similar to that of EA. The mass densities and molar concentrations of the linear carboxylates and EMC decreased with an increase in temperature. The plots of the mass density and molar concentration against temperature were slightly curved. The molar concentration was calculated from the mass density and the molar mass (M) according to the following equation:
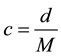 (1)
(1)
The molar concentration decreased in the following descending order: 2FEA > EFA > EA > EMC. The molar concentration dominated the order of the mass densities of EA and its derivatives: 2FEA > EFA (> EMC) > EA. As a result of the combined effect of the high molar mass and the high molar concentration, the mass densities of 2FEA and FEA were higher than that of EA.
Monofluorination of EA increased the mass and volume of the molecule. Since 2FEA and EFA are position
![]()
![]() (a) (b)
(a) (b)
Figure 2. (a) Mass densities (d) and (b) molar concentrations (c) of 2FEA, EFA, and EA single solvents as a function of temperature (θ) from 10˚C to 70˚C. Included in this figure for comparison are data for 1 mol dm−3 LiPF6 solution in EMC.
isomerism, the values of the relative molecular mass are equal to each other (Mr = 106.10). Taft’s steric substituent constants (ES) are a measure of steric effects of aliphatic substituents [20] . A large negative value of ES means a bigger steric effect. A fluoromethyl group (CH2F-) (ES = −0.24) is somewhat larger than a methyl group (CH3-) (ES = 0.00). The value of −0.24 is comparable with a chloromethyl group (CH2Cl-) (ES = −0.24). Interestingly, the molar concentrations of 2FEA and EFA were higher than that of EA in spite of the increased molecular volume. The molar concentration reflects both the strength of the attractive forces and the reverse of the molar volume. The volume of the monofluorinated EAs contracts to some extent because of the attractive forces between molecules, and consequently the molar concentration increases. The attraction of 2FEA and EFA molecules can be based on nonconventional weak intermolecular hydrogen bonding (CF−H・・・O = C or C−H・・・F−C) [20] as well as conventional dipole-dipole interactions. The weak hydrogen-bonding system does not exchange its proton and therefore it is no more a genuine hydrogen bond; it is an electrostatic attraction of positive charge on the hydrogen and negative charge on the organic fluorine or the organic oxygen [20] . A 2-fluoroethoxy (CH2FCH2O-) group in a 2FEA molecule can form the intermolecular hydrogen bonds (CF−H・・・O = C or C−H・・・F−C) more effectively than a fluoroacetyl (CH2FCO-) group in an EFA molecule. Therefore, the molar concentration of 2FEA became higher than that of EFA.
Figure 3 shows the temperature (θ or T) dependence of (a) relative permittivity (εr) and (b) viscosity (η) of 2FEA, EFA, and EA single solvents. In addition, this figure compares data for EMC, whose structure is similar to that of EA. The relative permittivity reflects the ease of dielectric polarization. The relative permittivity has a very significant effect on the strength of the interactions between ions especially in dilute solutions. The viscosity is regarded as internal friction based on intermolecular forces and affects electrolytic conductivity. Both the relative permittivity and the viscosity depend on the molar concentration. The relative permittivity of EA was more than twice that of EMC in spite of its low viscosity. Monofluorination of EA increased both the relative permittivity and the viscosity. It should be noted that the relative permittivity of EFA was higher than that of 2FEA, but that the viscosity of EFA was lower than anticipated from the relative permittivity: EFA > 2FEA > EA > EMC for the relative permittivity and 2FEA > EFA > EMC > EA for the viscosity.
The amount of orientation polarization of EFA was larger than that for 2FEA. An acetyl group (CH3CO-) is more electron-withdrawing and more rigid than an ethoxy group (CH3CH2O-). Monofluorination of the acetyl group can make the opposite large charges separated in the molecule, and hence induce the large bond moment. The net dipole moment of the EFA molecule may be larger than that of the 2FEA molecule. In contrast, the molar concentration of 2FEA was higher than those of EFA and EA because of the contraction in volume, as described above. The internal friction of 2FEA can increase under these circumstances, and accordingly the viscosity of 2FEA became higher.
The viscosity decreased exponentially with an increase in temperature, and plots of log10 (η/mPa s) vs. T−1 gave straight lines. The high translational kinetic energy allows intermolecular attractions to be overcome more easily, and the internal friction is reduced at high temperatures. The apparent activation energy for viscosity (Ea,η) is obtained from the relation proposed by Andrade [21] :
![]()
![]() (a) (b)
(a) (b)
Figure 3. (a) Relative permittivities (εr) and (b) viscosities (η) of 2FEA, EFA, EA, and EMC single solvents as a function of temperature (θ or T) from 10˚C (283.15 K) to 70˚C (343.15 K).
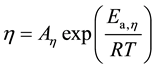 (2)
(2)
The apparent activation energy is determined to be 12.41, 12.02, 11.13, and 8.258 kJmol−1 for 2FEA, EFA, EMC, and EA, respectively. The viscosity and apparent activation energy of monofluorinated EAs were higher than those of EA and EMC. The apparent activation energy decreased in the same order as the viscosity: 2FEA > EFA > EMC > EA.
3.2. Electrolytic Conductivities of LiPF6 Solutions
Electrolytic conductivity of a solution is a key factor determining the internal resistance and rate performance of a battery. Figure 4(a) shows the temperature (T or θ) dependence of electrolytic conductivities (κ) of 1 moldm−3 LiPF6 solutions in 2FEA, EFA, and EA. Included in this figure for comparison are data for 1 mol dm−3 LiPF6 solution in EMC. The conductivities of the electrolyte solutions increased with an increase in temperature. The viscosities of these solvents decreased rapidly at elevated temperatures. The decrease in the relative permittivity at high temperatures would not so much influence the conductivity. The conductivity can be affected by the ionic mobility, the charge numbers of the ions, the concentration of the electrolyte, the degree of ionic dissociation, etc. The ionic mobility is related to the viscosity of the electrolyte solution. The degree of ionic dissociation of a lithium salt is set by the balance between the permittivity and Lewis basicity of the medium, depending on the concentration of the lithium salt [10] .
The electrolytic conductivity of an EA solution was more than twice that of an EMC counterpart. The relative permittivity of EFA was higher than that of 2FEA, whereas the viscosity of EFA was lower. Therefore, the high ionic mobility and the high degree of ionic dissociation produce a synergy effect on the conductivity of an EFA solution. The conductivity of an EFA solution was higher than that of a 2FEA counterpart over a temperature range of −5˚C to 70˚C. The introduction of a fluorine atom into an EA molecule may decrease the electron-pair donability of oxygen atoms in the -COO- moiety. This effect results in the decreased solvation of lithium ions and, consequently, in the lower degree of ionic dissociation. In particular, the monofluorination of the ethoxy group may decrease Lewis basicity more greatly than that of the acetyl group. The drop in the basicity of 2FEA may be produced by the nonconventional hydrogen bonds. Steric hindrance between the -COO- moiety and the 2-fluoroethyl moiety may also weaken the basicity of 2FEA. The plots of log10 (η/mPa s) vs. T−1 gave straight lines, as shown in Figure 3(b). In contrast, the plots of log10 (κ/mScm−1) vs. T−1 displayed upward curvature. The different features suggest that the conductivity does not vary inversely with the viscosity especially at elevated temperatures.
![]()
![]() (a) (b)
(a) (b)
Figure 4. (a) Conductivities (κ) of electrolyte solutions in 2FEA, EFA, EA, and EMC as a function of temperature (T) from −5˚C (268.15 K) to 70˚C (343.15 K). Electrolyte: LiPF6 (1 moldm−3 at 25 C). (b) The product of conductivity and viscosity (κη) as a function of temperature (θ) from 10˚C to 70˚C. We adopted the viscosities of the single solvents. Electrolyte solutions are the same as described in the caption of (a).
There was intersection of the two plots of conductivity against temperature: about 65˚C for 2FEA and EA solutions [12] ; and about 47˚C for EFA and EA solutions [12] . Consequently, the conductivity of an EFA solution was higher than that of the EA counterpart over a temperature range of 47˚C - 70˚C. The temperature range was wider than that observed for 2FEA (65˚C - 70˚C). The threshold temperature, where monofluorination of an organic solvent leads to an increase in conductivity, is also observed for 1 moldm−3 LiPF6 solutions in other linear carboxylates and linear carbonates: about 50˚C for 2-fluoroethyl propionate (2FEP) and ethyl propionate (EP) [13] ; about 8˚C for ethyl 3-fluoropropionate (E3FP) and EP [13] ; about 2˚C for ethyl 2-fluoropropionate (E2FP) and EP [13] ; about 45˚C for 2-fluoroethyl methyl carbonate (2FEMC) and EMC [5] ; and about 25˚C for ethyl 2-fluoroethyl carbonate (E2FEC) and DEC [7] .
Figure 4(b) shows the temperature dependence of the product of conductivity and viscosity (κη). These plots are different from the so-called Walden products. The κη was slightly decreasing at high temperatures. The decrease in the κη and the presence of the threshold temperature support the idea that the conductivity is not inversely proportional to the viscosity especially at elevated temperatures.
3.3. Electrochemical Stability and Cycling Efficiency of Lithium Anode
Linear sweep voltammetry (LSV) was carried out to investigate electrochemical potential windows of electrolyte solutions. Figure 5(a) shows linear potential sweep voltammograms obtained with a platinum electrode for 1 moldm−3 LiPF6 solutions in 2FEA, EFA, EA, and EMC at a scan rate of 5 mVs−1 at 25˚C. Monofluorination of EA increased the anodic stability, as easily predicted by the highest electronegativity of fluorine.
Most conventional measures for the electronic effect of substituents are provided as Taft (σ*) constants for substituents attached to aliphatic chains [20] . The electron-withdrawing inductive effect of a fluorometheyl group (CH2F-: Taft σ* = 1.10) is substantially stronger than that of a metheyl group (CH3-: Taft σ* = 0). Accordingly, the anodic stability of 2FEA and EFA was higher than that of EA. On the other hand, the anodic stability of 2FEA and EFA was slightly lower than that of EMC. We cannot determine the potentials for reduction decomposition of 2FEA, EFA, EA, and EMC in the potential range of 2 - 0 V. Small anodic peaks at about 5.8 V and cathodic peaks in a potential range of 2 V to 3 V can be ascribed to degradation products of LiPF6 or impurities in 2FEA and EFA.
Cycling efficiency of a lithium anode indicates coulometric efficiency achieved with the repetitiveness of the deposition and dissolution of lithium on a nickel electrode. Figure 5(b) shows the variation of the cycling efficiency with respect to the cycle number at 25˚C. We used 1 moldm−3 LiPF6 solutions in EC-2FEA, EC-EFA,
![]()
![]() (a) (b)
(a) (b)
Figure 5. (a) Linear sweep voltammograms obtained with a platinum electrode for electrolyte solutions in 2FEA, EFA, EA, and EMC at a scan rate of 5 mVs−1 at 25˚C. Electrolyte: LiPF6 (1 moldm−3). (b) Variation of cycling efficiency of lithium anodes with respect to the cycle number at 25˚C. Electrolyte solutions: 1 moldm−3 LiPF6 solutions in EC-2FEA, EC-EFA, EC-EA, and EC-EMC equimolar binary mixtures. The plating and the stripping current density were fixed at ±1 mAcm−2. The plated charge density was −300 mCcm−2, and the cut-off voltage was set to +1.0 V vs. Li|Li+ during the anodic stripping process.
and EC-EA equimolar binary mixtures as the electrolyte solutions. Included in Figure 5(b) for comparison are data for an EC-EMC equimolar binary mixture. The cycling efficiency was less than 20% in EC-EFA, EC-EA, and EC-EMC binary systems after the 12th cycle. The use of the EC-2FEA binary mixture remarkably suppressed the cycling efficiency fading. The morphology, thickness, density, and chemical composition of the surface film may be different from those of a surface film formed in a lithium anode|electrolyte solution interphase.
Figure 6 shows photographs obtained with scanning electron microscope (SEM) after the 10th deposition/ dissolution cycle of lithium. This figure shows that the morphology of the surface film was homogeneous in the EC-2FEA binary system (a). Furthermore, the surface film consisted of grains of small and regular size, and the thickness seemed to be relatively thin in this binary solvent system. In contrast, the morphology of the surface film was coarse and heterogeneous in the EC-EA binary system (c). Plank-type deposits, which might be lithium dendrite, were observed in this binary solvent system.
![]() (a)
(a)![]()
![]() (b) (c)
(b) (c)
Figure 6. SEM photographs obtained after the 10th deposition/dissolution cycle of lithium on a nickel electrode. Electrolyte solutions: 1 moldm−3 LiPF6 solutions in (a) EC-2FEA, (b) EC-EFA, and (c) EC-EA equimolar binary mixtures.
3.4. Performance of Li|LiCoO2 Coin Cells
We assembled 2025-type coin cells to evaluate the performance by a charge?discharge test. Figure 7 shows the evolution of discharge capacities of Li|LiCoO2 coin cells with respect to the cycle number at 25˚C. The coin cells were charged in a constant current (1 C)-constant voltage regime (4.2 V) regime for 1 h and then discharged to 3.0 V at a constant current of 1 C. The discharge capacity is expressed on the basis of the mass of LiCoO2 as a cathode. The discharge capacity in the EC-EA binary system decreased rapidly from 100 mAhg−1 to 20 mAhg−1 after the 20th cycle. The discharge capacity in the EC-2FEA binary system was considerably higher than that in the EC-EFA binary system. The use of 2FEA as the alternative low-viscosity solvent remarkably suppressed the discharge capacity fading at high cycle numbers. The cathodic decomposition of the 2FEA-based solutions on lithium anodes can form passive thin films containing adequate amounts of organofluorine compounds. Although the organofluorine compounds have not been identified, lithium ions may readily pass through the surface film. The possible organofluorine compound in the surface films is monofluorinated lithium alkylcarboxylate. It is known that lithium alkylcarboxylate (RCOOLi) is formed on electrode surfaces by
![]()
Figure 7. Variation of discharge capacities of Li|LiCoO2 coin cells with respect to the cycle number at 25˚C. Electrolyte solutions are the same as described in the caption of Figure 5(b). The coin cells were charged in a constant current (1 C) mode and further charged to keep constant voltage (4.2 V). Total charging time was set to 1 h. The coin cells were then discharged to 3.0 V at the constant current (1 C).
cathodic decomposition of nonfluorinated esters [22] .
4. Conclusion
The use of two monofluorinated EAs allows us to investigate the effect of position isomerism on the physical and the electrochemical properties. The molar concentration decreased in the following descending order: 2FEA > EFA > EA > EMC. The molar concentration dominated the order of the mass densities of EA and its derivatives: 2FEA > EFA (> EMC) > EA. The relative permittivity of EFA was higher than that of 2FEA, whereas the viscosity of EFA was lower than anticipated from the relative permittivity: EFA > 2FEA > EA > EMC for the relative permittivity and 2FEA > EFA > EMC > EA for the viscosity. The conductivity of 1 moldm−3 LiPF6 solution in EFA was higher than that in 2FEA. The conductivity in 2FEA was higher than that in EA above 65˚C. The use of an EC-2FEA equimolar binary solution remarkably suppressed the cycling efficiency fading. The use of 2FEA as a co-solvent greatly improved the discharge capacity of a Li|LiCoO2 coin cell after the 20th cycle. 2FEA is a prominent candidate for co-solvents in lithium secondary batteries.
Acknowledgements
We would like to thank Mitsubishi Chemical Group Science and Technology Research Center for a donation to this work.
NOTES
*Corresponding author.