Thermal Decomposition of Bamboo Phyllostachys Edulis Pretreated with Ionic Liquids-Water Mixtures ()
1. Introduction
Bamboo is a potential renewable resource major producing in China, South Korea, Japan and Eastern South Asia. The composition of bamboo differs from species, growing conditions, maturity, or harvest. Phyllostachys edulis, so-called “mosochiku” bamboo in Japan, a major bamboo species with low ash content make it an ideal precursor for activated carbon manufacturing. Thermal decomposition is one of the thermo chemical reaction methods to developing a clean energy which has been sought to substitute for fossil resources.
In developing and using biomass resources, the focus of those working on biomass deconstruction processes is thus the preparation of carbohydrate from biomass [1] -[3] . Recently, lignocelluloses biomass using ionic liquids (ILs) has been considerable for subsequent use in the production of biomass energy [4] . Adding water or other organic solvent to IL systems can reduce the process cost significantly if pretreatment effectiveness is not compromised. The ILs mixtures have been successful applied in enzymatic hydrolysis [5] -[7] . ILs-water mixtures proved to be highly effective for biomass pretreatment by selective removal of lignin and it was obtained objective results for bio-fuel [8] . However, there is no report on the utilization of ILs-mixtures pretreated biomass in the research field of pyrolysis. In this study, the characteristics of pretreated bamboo were analyzed by chemical analysis and X-ray diffraction analysis (XRD). An application involving the thermal decomposition behavior of pretreated bamboo was discussed by the thermogravimetric differential thermal analysis (TG-DTA). The thermal decomposition products were determined by the gas chromatography-thermal conductivity detector (GC-TCD).
2. Materials and Methods
2.1. Materials and Chemicals
Bamboo (Phyllostachys Edulis) samples were supplied by Koshikawa-Tikuzai Co. Ltd., Chiba, Japan. Samples having particle size between 0.25 and 0.50 mm were dried in a vacuum oven before the pretreatment process. The chemical composition of the investigated bamboo was list in (Table 1). The ionic liquids, 1-butyl-3-methy- limidazolium chloride (C8H15ClN2, [BMIM]Cl, purity ≥ 95%) was the product of BASF supplied by the Sigma-Aldrich Co. USA. 1-butyl-3-methylimidazolium tetrafluoroborate (C9H15BF4N2, [BMIM]BF4, purity ≥ 97%) was supplied by Wako Co. Ltd. All other chemicals in this study were reagent grade and were used without further purification.
2.2. Pretreatment Process
Bamboo powder samples were added into a flask containing ionic liquids and deionized water (mass ratio of bamboo powder to ILs is 1:10; molar ratio of ILs to Water is 1:1). Pretreatment experiment was heated and stirred in flask by oil bath at 130˚C for 30 min. In order to remove the ionic liquid from the pretreated sample, all the samples were washed by methanol and deionized water three times respectively. The pretreated bamboo was using in thermal decomposition experiment after dried in a vacuum oven at 105˚C for 24 h.
2.3. Chemical Composition Analysis
The industry analysis was measured according to the Japanese industrial standard method (JIS-M8812). The elemental analysis of bamboo samples had been performed using a CHN Corder (Model MT-5, Yanaco Co. Ltd., Japan). The EtOH/Benzene was used to determine the amount of organic extractives in the bamboo samples. The holocellulose content (cellulose + hemicellulose) were determined by Wise method. 17.5% NaOH method was used to determine the amount of α-cellulose. Sulfuric acid method was applied in Klason lignin. The acid- insoluble lignin was calculated as lignin content.
2.4. X-Ray Diffraction (XRD)
Crystalline structures were analyzed by an Ultima III X-Ray diffractometer (Rigaku Co. Ltd., Japan). Ni-filtered Cu Kα radiation (λ = 0.1542 nm) was generated from 40 kV voltage and 40 mA current. Intensity range was from 10˚ to 40˚ with 2˚/min scan speed for total X-ray diffraction (XRD) analysis experiment. The crystallite height 002 (I002) and amorphous height (Iam) were used to calculate the apparent crystalline index (apparent Cr.I.) and was calculated by Equations (1).
![]()
Table 1. Chemical composition of the bamboo samples
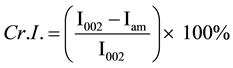 (1)
(1)
The apparent crystallite size L of the refection of plane was calculated from the Scherrer equation based on the width of the diffraction patterns.
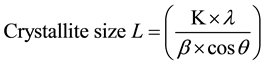 (2)
(2)
where,
K, the Scherrer constant of value 0.94;
λ, the X-ray wavelength (0.1542 nm);
β, the half-height width of the diffraction band;
θ, the Bragg angle corresponding to the planes.
The surface chains occupy a layer approximimately 0.57 nm thick so the proportion of crystallite interior chains and the interlayer distances d was calculated as the following Equation (3) and Equation (4):
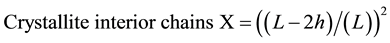 (3)
(3)
where,
L, the crystallite size for the refection of plane;
h, the layer thichkness of the surface chain is 0.57 nm.
 (4)
(4)
where,
λ, the X-ray wavelength (0.1542 nm);
θ, the Bragg angle corresponding to the planes.
2.5. Thermogravimetric-Differential Thermal Analysis (TG-DTA)
The thermal decompositions of samples were carried out in a TG-DTA (Model DTG-60, Shimadzu Co. Ltd., Japan) in order to calculate the yield of char and to survey the thermal decomposition behavior. About 20 mg of sample was placed on the scales in the apparatus. The sample was heated up to 900˚C at a constant heating rate of 5, 10 and 20˚C/min. The thermo gravimetric analysis was carried out under Ar atmosphere with a purge gas flow of 70 ml/min.
2.6. Gas Chromatography-Thermal Conductivity Detector (GC-TCD)
Thermal decomposition device used for samples thermal decomposition was present in Figure 1. It was composed of gas feeding system, pyrolysis system, tar decomposition system, condensable products trapping system and, gaseous products measurement system. The Gaseous products were measured by a GC-TCD/FID (Model GC-2014, Shimadzu Co. Ltd., Japan). H2, CO, CH4 and, CO2 were measured by a GC-TCD.
3. Results and Discussions
3.1. Characteristics of Ionic Liquid-Water Mixture Pretreated Bamboo
Ionic liquids have been used in attempts to dissolve the whole lignocellulosic biomass [9] . Most studies reporting solubilisation of lignocelluloses have used ionic liquids with imidazolium cations. Therefore, bamboo (phyllostachys edulis) samples were pretreated by [BMIM]Cl+water and [BMIM]BF4+water to obtain the insoluble component for subsequent applications. The water adding in this mixture has two purposes. The first is to prevent more bamboo dissolved in ILs as anti-solvent [10] , the other is expand the usage for large-scale application [11] . The raw bamboo powder, pretreated bamboo, ILs+water mixture and used mixtures were present in Figure 2. From the appearance of bamboo samples before and after the pretreatment in [BMIM]Cl+water and [BMIM]BF4+water mixtures, it is clear that more component from bamboo was dissolved in [BMIM]Cl− water
![]()
Figure 1. Thermal decomposition device using in this study.
![]()
Figure 2. Appearance of bamboo samples before and after the pretreatment in [BMIM]Cl+water and [BMIM]BF4+water mixtures.
mixture associated with the darker the color Figure 2. Although, no significantly differences on the appearance of pretreated bamboo.
The composition analysis of untreated bamboo and pretreated bamboo was determined in Figure 3. As a result, the amount of holocellulose including cellulose and hemicelluloses were increased in the pretreated bamboo from 72.1 to 78.9 and 75.9 in [BMIM]Cl+water and [BMIM]BF4+water, respectively. The Kalson lignin was decreased from 26.8 to 15.6 and 21.2 in [BMIM]Cl+water and [BMIM]BF4+water, respectively. The EtOH/ Benzene extractive has the similar change in result. In this study, more composition of Kalson lignin was dissolved in [BMIM]Cl+water than that in [BMIM]BF4− water. Most studies report reduces lignin content in the pretreated biomass. Lignin removel was reported to be between 17% and 65% for lignocelluloses such as softwood or hardwood. This is probably a sign that the soubilisation of lignin is susceptible to pretreatment conditions such as temperature, time and moisture content of the ionic liquid and biomass [1] . Differences in reactivity depending on the anion were observed. More strongly hydrogen bond-basic anions such as Cl−, Br− resulted in higher yields of the cleavage products than weakly basic anions. Less coordination anions such as 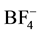 resulted in elimination of the hydroxyl methylene group. For cellulose,
resulted in elimination of the hydroxyl methylene group. For cellulose, 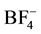 anions was eliminated due to their poor capability to dissolve any kind of cellulose. A good dissolution of cellulose may be obtained using halide based-ILs, especially with the chloride anion [12] . Therefore the small size and the strong electronegativity of the Cl− are obvious advantages. As a result, the composition of component of pretreated bamboo was changed.
anions was eliminated due to their poor capability to dissolve any kind of cellulose. A good dissolution of cellulose may be obtained using halide based-ILs, especially with the chloride anion [12] . Therefore the small size and the strong electronegativity of the Cl− are obvious advantages. As a result, the composition of component of pretreated bamboo was changed.
Cellulose crystallinity is often measured in connection with IL treatment which is of particular interest to the dissolution process. The X-ray diffractograms of untreated bamboo and pretreated bamboo was present in Figure 4 in order to understand the effect of ILs+water mixture on cellulose crystalline structure. A clear rising peak
![]()
Figure 3. Composition analysis of untreated bamboo and pretreated bamboo.
![]()
Figure 4. X-ray diffractograms of untreated bamboo and pre- treated bamboo.
in 002 planes was indicated that the composition amount of cellulose was increased in pretreated samples due to the composition amount of lignin and EtOH/Benzene extractive was decreased. Alter the structure of the cellulose fibrils was accelerating the subsequent hydrolysis. The result of interlayer distances showed that the treated bamboo had a less ordered structure than untreated bamboo (Table 2). This result suggested that the ILs+water affected the composition of bamboo and the cellulose crystalline structure containing in bamboo structure.
3.2. Thermal Decomposition Behavior of Pretreated Bamboo
Figure 5 shows the results of the thermogravimetric analysis preformed on the samples. Figure 5(a) gives the percentage of weight loss as a function of temperature, while Figure 5(b) presents the derivative thermogravimetric curves. The sample was dried in oven before used, thus no weight losee was observed around 100˚C. Then, the further thermal degradation takes place as a two-step process. First step is the degradation of hemicelluloses was observed at around 300˚C. A slight shoulder in the DTG curve for all samples studied. Second step is at about 350˚C the major degradation of cellulose occurs and a peak appears at the temperature corresponding to the max decomposition rate. Hemicellulose has a random amorphous structure and it was easily hydrolyzed [13] .
In contrast, the cellulose is a very long polymer of glucose units, and its crystalline regions improve the thermal stability of wood [14] . The degradation temperature of lignin was between 250˚C and 500˚C. In detail in Figure 5(a), the [BMIM]Cl+water shows that higher thermal stability than untreated bamboo. This behavior
![]()
Figure 5. TGA (a) and DTG (b) curves for the untreated bamboo and pretreated bamboo.
![]()
Table 2. The parameters obtained from the XRD analysis of the bamboo samples used in studied.
may be related to the higher crystallinity index and crystallite size in pretreated sample. The [BMIM]Cl+water has lower char than untreated bamboo and [BMIM]BF4+water. This result suggested that smaller crystallite size in [BMIM]Cl+water sample than that in untreated bamboo. The similar behavior was observed in other heating rate was show in Table 3. The temperature (Tpeak) of maximum decomposition was increased associated with the heating rate.
3.3. The Products Accompanied by Thermal Decomposition
Gaseous products profiles of bamboo thermal decomposition was were measured by a GC-TCD were described in Figure 6; the tar as products at 900˚C was present in Table 4. The CO and CO2 has prominent peak appears at the temperature corresponding to the max decomposition rate was associated with the decomposition of cellulose.
It is interesting that around twice molar quantity of CO was produced than that from untreated bamboo. The amount of H2 were decreased in the pretreated bamboo due to the lignin content was dropped in the pretreated samples. The amount of tar were decreased in the pretreated bamboo from 0.544 to 0.430 and 0.444 in [BMIM]Cl+water and [BMIM]BF4+water at 900˚C, respectively was presented in Table 4. The pretreated bamboo with [BMIM]Cl+water mixture was tend to produce the more gaseous products, which associated with the decomposition rate. The behavior of more gaseous products and less tar in the thermal decomposition product can be attributed to ILs-water pretreatment process. The study on thermal decomposition of bamboo would be helpful to better design manufacturing process of bamboo composite or bio-energy, made from bamboo by thermal chemical conversion methods such as gasification and pyrolysis. The pretreatment methods could disrupt the biomass structure so that the carbohydrates become accessible. One of methods is the deconstruction of biomass with the aid of ionic liquids. Alter the structure of the native cellulose fibrils and accelerate the subsequent application. On the other hand, the recovery of major components as co-products such as lignin, which partly dissolved in IL mixture, will be another issue to overcome. The potential of IL mixture pretreatment method applied in thermal chemical conversion methods such as gasification and pyrolysis could be expected.
![]()
Figure 6. Gaseous products profiles of bamboo thermal decomposition, (a) H2; (b) CO; (c) CH4 and (d) CO2. TGA (a) and DTG (b) curves for the untreated bamboo and pretreated bamboo.
![]()
Table 3. Tpeak of thermal degradation decomposition and mass loss of the different samples.
![]()
Table 4. The thermal decomposition products obtained from bamboo samples at 900˚C/%・g−1.
4. Conclusion
The ILs+water pretreatment process could affect the composition of bamboo and the cellulose crystalline structure in bamboo (phyllostachys edulis) samples. The lignin content and EtOH/Benzene extractive was conducive to dissolved in ILs+Water mixture. It was around twice molar quantity of CO was produced than that from untreated bamboo. The pretreated bamboo with [BMIM]Cl+water mixture was tend to produce the more gaseous products, which were associated with the decomposition rate. The behavior of more gaseous products and less tar in the thermal decomposition products can be attributed to ILs+water pretreatment process. It may be considerable for subsequent use in the production of biomass energy from waste bamboo.
Acknowledgements
The research was supported by the special funds for Basic Researches (B) (No. 22303022) of Grant-in-Aid Scientific Research of the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
NOTES
*Corresponding author.