A Study on Ethanolysis and Methanolysis of Coconut Oil for Enzymatically Catalyzed Production of Biodiesel ()
1. Introduction
The demand for renewable fuels has recently been much intensified. In this scenario, biodiesel arises as an alternative to petroleum products, aiming to reduce pollutant emissions in the atmosphere.
Biodiesel is the name of a clean-burning fuel produced from domestic renewable resources. It does not contain petroleum, but can be added to it forming a blend [1] . It can be used in a compression-ignition engine (diesel) without need for modification. Biodiesel is simple to use, biodegradable, non-toxic and essentially free of sulfur and aromatic compounds [2] .
To produce biodiesel, vegetable oils are mixed with short-chain alcohols (ethanol or methanol) and then stimulated by a catalyst. This chemical process is known as transesterification, where glycerin is separated from the fat or oil. The process generates two products: esters (the chemical name for biodiesel) and glycerin (a valuable product in the soap industry). The oil is separated from the glycerin by filtration process [3] .
High-quality biodiesel must be produced following strict industry specifications, as the ASTM D6751 at international level. In USA, biodiesel is the only alternative fuel to obtain full approval in the Clean Air Act of 1990, which is authorized for sale and distribution by the USA Environmental Agency (EPA) [4] .
A very appropriate nomenclature has been used internationally to identify the concentration of biodiesel in the blend, defined as BX, wherein X refers to the percentage by volume of biodiesel. Therefore, B2, B5 and B20 refer to fuels at concentrations of 2%, 5% and 20% of added biodiesel, respectively [5] .
Although soybean oil is the most widely used in biodiesel production, other oilseed plants with a higher oil content, such as peanut, sunflower, corn, canola (rapeseed), castor oil and cotton have been reported in the literature as favorable for the production of biodiesel [6] - [8] . In this context, coconut oil has been identified as an important potential source of raw material for the local production of biodiesel in the northeast of Brazil [9] .
In the transesterification of vegetable oil, a triglyceride reacts with an alcohol in the presence of a catalyst, which may be an acid, a strong base or an enzyme, producing a mixture of alkyl esters of fatty acids and glycerol. The total process is a sequence of three consecutive and reversible reactions, where diglycerides and monoglycerides are formed as intermediates. For a complete stoichiometric transesterification, a 3:1 molar ratio of alcohol to triglyceride is required. Due to the reaction’s reversible character, a transesterification agent (alcohol) is usually added in excess, thus contributing to increasing the ester yield, and to enabling its separation from the glycerol formed [10] .
Currently, the commercial production of biodiesel occurs via a chemical route, but the enzymatic process has attracted the interest of the scientific community. Although the biodiesel production through enzymatic transesterification has not yet been commercially developed, advances in this process have been reported in papers and patents. The common aspect of these studies is the optimization of reaction conditions (solvent, temperature, pH, type of microorganism that produces the enzyme, immobilization technologies, etc.), in order to establish the characteristics for industrial applications [11] .
However, once the enzymatic process is optimized, it may present some advantages over the chemical process (Table 1).
Methanol is used as the acyl group receptor on biodiesel production by transesterification of oils or fats using lipases, according to most recent studies found in the literature. Methanol is more reactive, and produces more volatile esters and it is cheaper on the international market, when compared with other short chain alcohols [12] .
However, some authors are considering other alcohols, such as ethanol, propanol, isopropanol and butanol for the production of biodiesel component esters, due to methanol’s toxicity and by the fact that it is produced from petroleum. Ethanol is preferred because it is considered renewable [13] .
The objective of this work was to study the variables that influenced the transesterification of coconut oil in the production of biodiesel catalyzed by immobilized lipase. The transesterification was performed in sealed glass reactors stirred at 200 rpm and catalyzed by the commercial immobilized lipase Novozym 435 using ethanol and methanol. Assays were carried out in accordance with an experimental design where the variables were temperature (40˚C - 60˚C), enzyme concentration (3% - 7%), ratio oil-alcohol (1:6 - 1:10) and type of alcohol (ethanol and methanol).
![]()
Table 1. Advantages and disadvantages of the chemical and enzymatic processes in biodiesel production.
2. Materials and Methods
2.1. Materials
The samples of crudecoconutoil were provided by SOCOCO Coconut Food Industry, located in Maceió, Brazil. According to the supplier, the extraction of coconut oil was made by mechanical pressing of the dehydrated pulp.
Enzymes used in this study were provided by Novozymes, Latin America (Brazil). Other reagents used were of analytical grade.
2.2. Characterization of the Transesterification Products
To determine the biodiesel yield (%), the characterization of methyl or ethyl esters was carried out using gas chromatography (GC). Transesterification products were analyzed by gas chromatography using a VARIANCP- 3800 instrument equipped with a FID detector (Flame Ionization Detection) and a short capillary column (2.3 m). The temperature of the detector was 250˚C and the injector was 240˚C. The oven had temperature programmed from 150 to 260˚C at a heating rate (ramp) of 10˚C/min. Tricaprylin was used as the internal standard and hydrogen of high purity (99.95%) was used as the carrier gas.
The analyzed sample was prepared by mixing 0.15 mL of biodiesel previously purified with 1 mL of standard solution (tricaprylin plus hexane in desiccator). A 1 μL aliquot of the sample was then injected into the chromatograph, with a 10 mL glass syringe.
The yield calculation in esters was carried out based on the masses and areas under the peaks corresponding to the methyl or ethyl esters and to the internal standard, using Equation (1):
 (1)
(1)
where:
mp = internal standard weight (0.08 g);
Ab = sum of the peak are as referring to the esters in the sample (peak detected between 8 and 13 min);
f = response factor (0.78);
Ap = peak area referring to the internal standard (tricaprylin plus hexane- peak detected between 15 and 18 min);
mb = sample weight (0.15 g).
The conversion analyses were performed in duplicate. The average conversion for each experiment was also calculated.
3. Experimental Stage
3.1. Enzymatic Transesterification
The reactions, in laboratory scale, were carried out in 250 mL Erlenmeyer flasks closed with glass lids. The commercial lipase in pre-established concentrations was added to the oil-alcohol mixture. The flasks were then incubated in a rotating chamber at 200 rpm at a controlled temperature. The amount of coconut oil used was held at 25 g.
After the elapsed reaction time, the sample was filtered (in order to retain the enzymes for subsequent recovery and use) and then taken to a rotary evaporator at 90˚C at 40 rpm, to evaporate the alcohol.
After this procedure, the resulting reaction mixture was placed in a separatory funnel, where the upper phase, concentrated in methyl or ethyl esters (biodiesel), was separated from the lower phase containing the remaining reaction products (mono and diglycerides, glycerol and impurities), as well as the unreacted substrates (alcohol and oil).
3.2. Experimental Design
The experimental design is a very useful tool for laboratory research. In addition to allowing research with several variables, it also enables an easy determination of the variation effects, since determining the influence of one or more variables over the others is a common problem.
A full factorial experimental design with two levels and four variables was performed to determine the experimental conditions that maximize the synthesis of biodiesel resulting from the reaction and to evaluate the influence of selected variables.
The variables studied at this stage were: oil-alcohol molar ratio, temperature, enzyme percentage and alcohol type. Based on preliminary studies, the agitation was kept constant, since it has been verified that no change results when increasing it from 200 to 500 rpm [14] . The reaction time was set at 24 hours. The limitations associated with each variable are shown in Table 2.
The experimental matrix for the 24 factorial design is presented in Table 3.
Through the obtained experimental data, an empirical model was developed with the variables coded by the letter x. This model was used to estimate the yield of coconut oil biodiesel by using coded values of the variables here studied. These coded values must belong to the interval −1 ≤ x ≤ +1, which was considered in this work.
Therefore, the following mathematical model, Equation (2), for the prediction of desired responses was adopted:
 (2)
(2)
where:
y = percentage of the variable of interest (yield);
x = variable that shows the factors in coded scales. As in the present work only the low and high levels are being considered, x assumes −1 and +1 values;
β = parameters which values will be determined.
For practical purposes, the β0 estimate is the general average of the responses, while the estimates of the other parameters were obtained from the regression table given by the STATISTICA7.0.
![]()
Table 3. Experimental design matrix.
4. Results and Discussion
4.1. Transesterification Reactions
The experimental data obtained in the enzymatic transesterification of coconut oil using the commercial lipase Novozym 435 as a catalyst are shown in Table 4. Yield data referred to a 24-hour reaction. It is noteworthy that the experiments were done induplicate at each point.
Table 4 shows that the highest yield (80.5%) was obtained at the higher temperature (60˚C), enzyme concentration (7%), molar ratio of oil:alcohol (1:10) and using ethanol.
Once the yield was determine, a statistical model was built for representing the experimental data, assessing the significance of the variables, and evaluating possible interactions between them.
The statistical model for calculating the yield of coconut biodiesel can be represented by Equation (3):
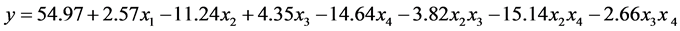 (3)
(3)
where:
The regression parameters 2.57, −11.24, 4.35, −14.64, −3.82, −15.14, and −2.66 were obtained from Table 5, and the parameter 54.97 is the mean of all responses obtained from the experiments:
y is the yield (response) estimate percentage;
x1 is the coded variable of enzyme concentration which belongs to the interval −1 ≤ x ≤ 1;
x2 is the coded variable of temperature which belongs to the interval −1 ≤ x ≤ 1;
x3 is the coded variable of molar ratio which belongs to the interval −1 ≤ x ≤ 1;
x4 is the coded variable of alcohol type which belongs to the interval −1 ≤ x ≤ 1.
The analysis of variance (ANOVA) (Table 6) shows that the model used in this study is statistically significant and suitable to represent the relation between response and the significant variables, because the coefficient of determination is satisfactory (R2 = 0.98). This value indicates that 98% of the variation of the yield can be attributed to the independent variables and only 2% of the total variation cannot be explained by the model, suggesting that there was a good fit of the model to the experimental data.
Linearity and good fit of the model are shown in Figure 1 with a confidence interval of 95%.
To a significance level of 5%, the obtained results indicate that enzyme concentration, temperature, molar ratio, type of alcohol, as well as interactions between temperature and molar ratio, temperature and type of alcohol, and molar ratio and type of alcohol are significant. These significant values (p < 0.05) are best viewed through
![]()
Table 4. Yields obtained in the transesterification of coconut oil.
![]()
Table 5. Regression coefficients for the biodiesel yield.
![]()
Table 6. Analyses of variance (ANOVA).
R2 = 0.98.
![]()
Figure 1. Confidence interval between the model and the experimental data at 95%.
the Pareto chart in Figure 2:
The values present in the Pareto chart represent the individual influence of each variable on the system response (the yield, in this case) and are interpreted as follows: The reaction yield decreases 30.29% on average when the temperature changes from the lower (40˚C) to the higher level (60˚C) and when ethanol (lower level) is exchanged for methanol (higher level);
The reaction yield decreases 29.27% on average when using methanol (higher level) instead of ethanol (lower level);
The yield of the reaction decreases 22.49% on average when the temperature changes from the lower (40˚C) to the upper level (60˚C);
The yield of the reaction increases 8.7% on average when the molar ratio changes from the lower (1:6) to the upper level (1:10);
The reaction yield decreases 7.64% on average when the temperature changes from the lower (40˚C) to the top level (60˚C) and the molar ratio changes from the lower (1:6) to the top level (1:10);
The reaction yield decreases 5.32% on average when the molar ratio changes from the lower (1:6) to the upper level (1:10) and when ethanol (lower level) is replaced by methanol (top level);
The reaction yield increases 5.15% on average, when the enzyme concentration changes from the lower (3%) to the upper level (7%).
The interactions between enzyme concentration and type of alcohol, enzyme concentration and molar ratio, and enzyme concentration and temperature had no significant effects, since p > 0.05. Although not significant, this result provides very interesting and attractive information. One of the problems involving enzymatic catalysis is the enzyme costs. A smaller amount of enzyme would make the process more feasible and appealing. The results obtained in this study show that the yield is not significantly influenced when 3% or 7% of enzyme are used. Hence, coconut oil biodiesel can be produced using enzyme concentrations below 7%, and still provide good yields.
4.2. Influence of Reaction Parameters
The higher the enzyme concentration, the greater the reaction yield [15] . In this study, the enzyme concentration had a positive effect, showing a higher yield when a greater amount of enzyme was used. However, when the assays 7 and 8 are compared it is notice that using 3% of enzyme, the yield did not decrease significantly; this variable can be optimized in future studies, keeping the other variables in the best conditions.
![]()
Figure 2. Pareto diagram with a significance level of 5%.
The molar ratio of substrate is a variable with great influence on the biodiesel synthesisreaction. Excess alcohol in the 3:1 stoichiometric ratio is used to ensure a high reaction rate and minimize diffusion limitations. However, excessive levels of alcohol can inhibit the enzyme activity, thus decreasing its catalytic activity during the transesterification reaction. Therefore, a high oil-alcohol ratio means a higher medium polarity produced by alcohol and water. This inactivates the enzyme and consequently the alkyl esters yield [16] .
As two types of alcohol were used in this study, these influences were evaluated separately.
When using ethanol, the molar ratio had a positive effect, demonstrating no enzyme inhibition for alcohol excess. This result corroborates [17] who observed similar effects when using castor oil.
Regarding the methanol, the molar ratio had negative effect, showing that the smaller molar ratio (1:6) yielded better results. This can be explained by the fact that there is a greater deactivation of enzymes by this alcohol [18] .
The reaction rate increases with temperature. In this work, at a temperature range between 40˚C and 60˚C, enzyme activity increased with temperature. However, when the temperature interacts with an alcohol, this behavior cannot be generalized. Temperature affects differently each type of alcohol. When using ethanol, the temperature had a positive effect, showing no enzyme inhibition at high temperatures.
When ethanol is replaced by highly volatile methanol, a significant yield drop was noticed. This can most likely occur due to the boiling point of methanol (64.70˚C) to be very close to the maximum temperature used (60˚C).
4.3. Influence of Alcohol Type
Methanol used in the transesterification reaction did not produce good yields when compared to ethanol. The results obtained for this alcohol corroborate those found in the literature. Methanol is a highly hydrophilic solvent, thus being able to solubilize and remove the essential water layer surrounding the enzymes, which can lead to a loss of catalytic activity of the lipase [16] - [19] . According to [19] , the poor results obtained with methanol may be related to the fact that the material used to immobilize enzymes (acrylic resin) can absorb polar compounds such as methanol. When the alcohol concentration in the mixture is high, it forms droplets that bind with the resin particles. The binding of alcohol molecules to the enzymes blocks the reaction (input) with triglycerides, resulting in low yields.
The use of ethanol in transesterification reactions to obtain coconut biodiesel had a positive effect. This result is very interesting and attractive, because ethanol has some advantages in relation to methanol, including: lower toxicity, renewability and availability in the region of this study.
Once discovered the best conditions for the production of biodiesel within the range studied in this work, it was possible to obtain the response surface for the yield using molar ratio and temperature as independent variables (Figure 3). The other two variables (enzyme concentration and type of alcohol) were fixed at their best results (7% and ethanol). Figure 3 shows that by using ethanol and 7% of enzyme, the yield increases when a molar ratio of 1:10 (+1) at 60˚C (+1) is used.
The best yield obtained in this study was 80.5% with 60˚C, using 7% of the enzyme, molar ratio oil-alcohol 1:10 and ethanol and this yield is too low when compared with alkali-catalysed process and the reaction rates are appreciably slower. Although, enzyme-based process has other advantages such as the capable of minimizing saponification problems due to a relatively high free fatty acid content [20] . Others papers that investigated biodiesel from coconut oil enzymatically catalyzed has obtained yield values between 35 and 90% [20] - [22] .
5. Conclusions
Biodiesel production from coconut oil was conducted by enzymatic catalysis in the presence of methanol or ethanol. The process conditions (temperature, molar ratio oil-alcohol, enzymeconcentration and type of alcohol) were varied in order to check their influence. To this end, a full 24 factorial design was performed.
This experimental design has proven efficient to study the influence of the process variables. The effects of temperature, type of alcohol, molar ratio, enzyme concentration and interactions between temperature and molar ratio, temperature and type of alcohol and the molar ratio and type of alcohol were very significant. The model was adjusted to the factorial design responses, showing the characteristics of a significant model under the statistical point of view, and the model has also predictive characteristics within the range studied for each variable.
The best yield in this study (80.5%) was obtained with 60˚C, using 7% of the enzyme, molar ratio oil:alcohol
![]()
Figure 3. Response surface to yield in biodiesel as a function of the molar ratio and temperature.
of 1:10 and ethanol. The results obtained when using methanol were not as significant when compared with ethanol. The results obtained using ethanol were very attractive, because ethanol was renewable and easily obtained in the region of this study.
NOTES
*Corresponding author.