Analysis and Formation of Hazardous Substances from Surfactant-Grafted Polyacrylamide Powder for Oilfield Production ()
1. Introduction
The Enhanced Oil Recovery (EOR) techniques have widely been used in oilfields, which mainly include the polymer flooding (PAM), SP flooding (Polymer-Surfactant) and ASP flooding (Alkali-Polymer-Surfactant). There are varied degrees of problems in the application of the above flooding, such polymer flooding remains high interfacial tension between the fluid and crude oil, basically no solubilization and emulsification capacity for crude oil, no polymer with the anti-salt, anti-bacterial and anti-oxidation function, and no preparation with sewage. The components of chemicals in Binary or ternary chemical flooding have quite difference in the diffusion and migration in the reservoir, exhibited in the “chromatographic effect” while no “synergies effect”, results in the low enhanced oil recovery. In addition, the strong base additives also led to the severe scaling in many facilities during the recovery operation. Therefore, there are some inconveniences to the production [1] - [3] .
A new type of compound has been required for the above reason. Generally, basic polyacrylamide (PAM), which is simple, long and repeated chain (CH2-CH2-CONH2) polymer with the appropriate stability, is employed for the popular polymer flooding. Recently, Surfactant-grafted Polyacrylamide (Referred to as S-PAM), which combines the advantages of polymer and surfactant, has been developed for the field test. It collects the advantages of enhancing the anti-shearing, reducing the flow ratio, expanding the sweep efficiency, reducing the interfacial tension, improving the solubilization and emulsifying capacity, etc. [4] -[7] .
Relevant data reveal that Surfactant-grafted Polyacrylamide (S-PAM) has a framework constituted by ordinary polyacrylamide hydrocarbon chains. By grafting and copolymerizing the functional monomers and functional group to the lateral groups of the molecular chains, the original polymer hydrocarbon chains can be converted into the hydrocarbon chains containing a large number of surfactants. Therefore, the aqueous solution of S-PAM is provided with the physical and chemical properties of the polymer and surfactant, as well as the new properties which are absent in the two agents. Generally, surfactant reduces the interfacial tension between oil and water in oil-water two-phase system to improve oil displacement efficiency. The S-PAM disperses the nonpolar substances in non-water system (including crude oil, coal power, cement, pigment, and so on) into emulsion and thus overcomes the application limitations of conventional surfactants in non-aqueous system.
The oil displacement experiments using S-PAM in multi-blocks revealed that in-use S-PAMs differ greatly from ordinary polymers. Based on the structure, chemical properties and manufacturing technique, small molecule volatile gases may be adsorbed in the surface of S-PAM, such as H2S, HCl, SO2, HCHO, NH3, Cl2, HF, CO, NO2, O3, PH3, etc. This study detected gases release of two commonly used S-PAM powders in oil field through chemical analysis and instrumental analysis methods.
2. Experimental
2.1. Materials and Instruments
Two origins of S-PAM were used in this experiment: Daqing Refining & Chemical Company S-PAM (Lianhua type III), which is called Lianhua S-PAM; Shanghai Haibo company S-PAM (Haibo type III), which is called Haibo S-PAM; The main instruments have ammonia gas meter (Z-800), Formaldehyde gas sensor (Formaldemeder-htv) and multigases detector (USA).
2.2. Identification Methods and Principles of Hazardous Gases
Two S-PAM powders in the plastic wrap sealed containers were prepared for measurement respectively. The gases were measured by the sensors of detectors which were inserted into the glass container from a hole of plastic wrap, or imported into the solution by needle. Gases emissions of two S-PAM powders were measured at room temperature respectively. The highest content of gases was measured, which was converted to mass of gas evolution per gram of solid S-PAM.
2.2.1. Identification of Formaldehyde
Gases emissions of two S-PAM powders within the container were measured by Formaldehyde gas sensor (Formaldemeder-htv) at room temperature respectively.
2.2.2. Identification of Ammonia
Ammonia emissions of two S-PAM powders within the container were measured by ammonia gas meter (Z-800) at room temperature respectively.
2.2.3. Identification of Hydrogen Sulfide
The principle is that hydrogen sulfide and copper sulfate solution generated a black precipitate, then H2S was detected.
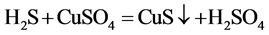
Gases emissions of two S-PAM powders within the container were imported into the copper sulfate solution, at room temperature, and the phenomenon was observed repeatedly to see whether a black precipitate (CuS) formed.
2.2.4. Identification of Hydrogen Chloride
The principle is that white precipitate is produced by the reaction of hydrogen chloride with silver nitrate, and then HCl was detected.
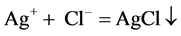
Gases emissions of two S-PAM powders within the container were imported into the silver nitrate solution, at room temperature, and the phenomenon was observed repeatedly to see whether a white precipitate (AgCl) formed.
2.2.5. Identification of Sulfur Dioxide
The principle is that sulfur dioxide solution can make magenta fade, and then SO2 was detected.
Gases emissions of two S-PAM powders within the container were imported into magenta solution (diluted), and the phenomenon was observed repeatedly to see whether magenta faded.
2.2.6. Identification of Hydrogen Phosphide
The principle is that different concentrations of phosphine gas in case of silver nitrate generated light brown or dark brown silver phosphate ,which emerged light brown or dark brown precipitate (Ag3P), then PH3 was detected.
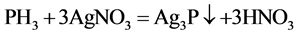
Gases emissions of two S-PAM powders within the container were imported into the silver nitrate solution at room temperature, and the phenomenon was observed repeatedly to see whether a light brown or dark brown precipitate (Ag3P) formed.
2.2.7. Identification of Other Gases
Gases emissions of two S-PAM powders within the container were measured directly by multigases detector (USA) at room temperature for the presence of Cl2, HF, CO, NO2, O3, etc.
3. Results and Discussion
3.1. Identification Results of Gases
Chemical methods and instruments were used the above method for qualitative identification, detecting the presence of H2S, HCl, SO2, HCHO, NH3, Cl2, HF, CO, NO2, O3, PH3, etc. Gases emissions of two S-PAM powders were measured at room temperature respectively. The highest content of gases was measured, which was converted to mass of gas evolution per gram of S-PAM powder. The test results were shown in Table1
Through qualitative identification of two S-PAM powders for eleven gases, only ammonia was detected.
3.2. Mechanism of Ammonia Release
S-PAM is Surfactant-grafted PAM, the structure and nature of which show some changes after grafted. Pyrolysis reaction of PAM has been adequately studied under the thermal effect. The powder PAM is thermally stable below 200˚C, which has only slight loss of quality in a relatively short period of time. The mass loss is mainly due to loss of adsorbed water or other volatile impurities. Pendant groups of PAM exploded over 200˚C - 220˚C. Then molecular chain of PAM breaks at higher temperatures. PAM is easily hydrolyzed at lower temperature (40˚C - 60˚C) in alkaline conditions. Acidic hydrolysis of PAM is required high temperatures, which is slower than the alkaline hydrolysis [8] . According to previous experiments, the ammonia release of powder S-PAM was measured at room temperature to 80˚C. And ammonia release of S-PAM solution was measured at room temperature. The chemical components and stereo structure should be modified in the some extension after the basic PAM is grafted. The harmful ammonia gas was generated during the operation of S-PAM or high temperature.

Table 1. Test results of toxic and hazardous gases.
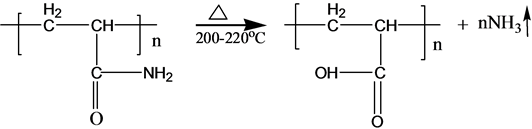
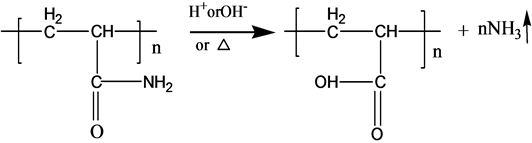
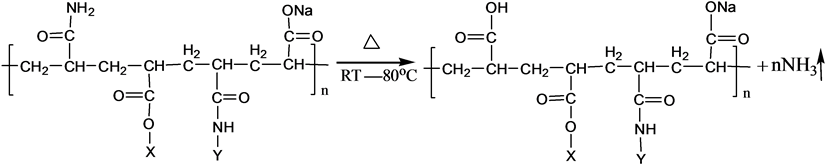
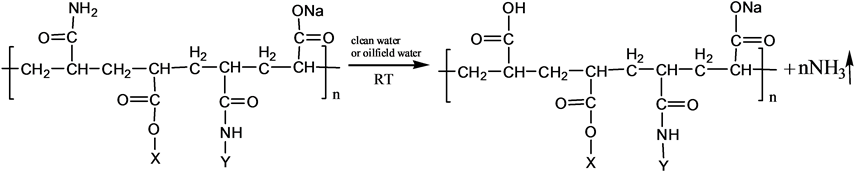
4. Conclusion
Through qualitative identification of two S-PAM powders for eleven gases, only ammonia was detected; H2S, HCl, SO2, HCHO, Cl2, HF, CO, NO2, O3, PH3 were not detected.