Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation ()
1. Introduction
The presence of the toxic metals generated by mineral processing in industries causes a major hazard to the water environment [1] . Thus, serious regulations are required to establish in many countries to remove effectively the toxic metal ions from the waste waters prior to discharge into natural environment. Also, metal ions are non-biodegradable materials and excessive levels can be damaging to human organism. The contamination of metal ions has a serious influence on the public health. Therefore, the elimination of metal ions from industrial waste to water is necessary to solve the environment of water cleaning. During the last few years, the common methods available to remove metal ions from waste water are coagulation, chemical precipitation, ion-exchange, and reverse osmosis [2] . In addition, the adsorption techniques present excellent qualities for treating industrial waste waters containing metal ions, when solid adsorbents are employed for recovery of metal ions as lead, copper, cadmium, nickel and zinc [3] [4] . Therefore, in the approach to replace the conventional adsorbents, geopolymers are a new strategy for decontamination of metal ions from waste water, which are composed of silica and alumina similar to zeolite material [5] - [9] . For this reason, the geopolymers has begun to develop as adsorbent materials in the process of removal metal ions from the waste water as an alternative to the industrial sector [9] . It is known that geopolymers are alkali activated aluminosilicates, consisting of a solid reactive component that contains SiO2 and Al2O3, for example, fly ashes, active clays, pozzolanas and slags [10] [11] . The alkaline activation solution for geopolymerization process contains alkali hydroxides, silicates, aluminates, carbonates, and sulphates or combinations thereof [10] . Several researches used metakaolin as ideal raw material for manufacture geopolymers [12] [13] because of its high reactivity and purity compared to other clays [14] - [16] . Here, zeolite which presents crystalline nature is well known as representative solid adsorbent for metal ions. Noticing is very meaningful that geopolymers have an amorphous three-dimensional structure constituted by SiO4 and AlO4 tetrahedra and can be prepared at lower temperature than zeolites. These geopolymers would be expected to have the unique properties as well as zeolite adsorbents. Furthermore, the geopolymers became an important subject in the following properties: compressive strength of the matrix and resistance to acid attack, freezing and heat thaw cycles. Such characteristic makes them interesting products for adsorbents as used with concrete replacements in various environments. If geopolymers can actually remove metal ions from waste water via adsorption, the regenerated matrix could become new approach for several industries. Consequently, this affects both the environment and societies positively [17] - [19] . However, existing literature on the adsorption of heavy metals using geopolymers is very little. Among them, Xu et al. reported the conversion of fly ash to geopolymer was investigated under different conditions for adsorbents. It was paid great attention as a potential material that geopolymers showed removal of Cd, Ni, Pb (II), Cu (II), phosphate, NOx, boron, fluoride, radionuclide of 137Cs and 90Sr, and dyes [20] [21] . However, the details about geopolymer adsorbents still are not well known at this time. In the present work, the main aim is synthesis of amorphous geopolymers from metakaolin and silica fume in order to use the materials as adsorbents for decontamination of heavy metal ions including Cs+ and Pb2+. The preparation and metal adsorption of the geopolymers were focused in different Si and Al amounts in the resultant matrix. The adsorption behavior was examined in a mixture of aqueous solution in detail for targeted separation of Cs+ and Pb2+ ions.
2. Experimental
2.1. Materials and Geopolymer Synthesis
The metakaolin (MK) was produced by the calcination of the kaolinite [Al2Si2O5(OH)4] at 700˚C for 5 h [22] [23] and was used as Al2O3 source for the synthesis of geopolymers. Also, silica fume AEROSIL 380 purchased from EVONIK industries was used. The role of adding silica fume was to support the sufficient amount of SiO2 on the resulting geopolymers. For the alkaline activator, aqueous sodium hydroxide (NaOH) was mixed with the silica fume (SiO2) using a ratio of Na/Si = 0.6. Figure 1 shows the illustration of synthesis protocol of metakaolin based geopolymer. MK was mixed in aqueous NaOH 8 M using a ball mill for 5 h with ratio of H2O/Na2O = 20. Here, the molar ratio between SiO2 and Al2O3 was changed at Si/Al = 1, 2, 3, 4, and 5. Then, the mixed pastes were casting into 20 mm latex cube molds and vibrated for 5 min to release the airs bubbles. The geopolymer pastes were cured at 80˚C for 12 h to start condensation reaction. Upon removal from the molds, the resultant geopolymers were placed in an oven at 200˚C for 12 h in order to complete the polycondensation. Principal composition of raw materials for geopolymers is presented in Table 1.
2.2. Characterization of Resultant Metakaolin Based Geopolymers
The obtained geopolymers were washed several times by deionized water in order to remove the excess of sodium hydroxide. After drying, the samples were crushed and sieved using a 120 mesh to control a particle size range. Before the adsorption tests of metal ions, the powder samples were characterized by FT-IR spectroscopy for determination of molecular vibration of the geopolymers, X-ray diffractometer (XRD) for crystal structure determination, X-ray fluorescence (XRF) in order to know chemical composition of principal components and Scanning electron microscopy (SEM) was used for evaluation of geopolymers morphology. The Brunauer-Emmer-Teller (BET) surface area was measured by a N2 adsorption―desorption after drying at 200˚C and the
![]()
Figure 1. Synthesis protocol of metakaolin based geopolymer.
![]()
Table 1. Chemical composition of the metakaolin-based geopolymer adsorbent.
zeta potential was measured on a potentiometer (ELSZ1NGK Photal Otsuka Electronics instrument) in the absence and presence of 5 wt% and 10 wt% of NaCl at pH 5.
2.3. Adsorption Tests of Geopolymers for Heavy Metal Ions
The adsorption experiments of the geopolymers were performed by the batch system at 25˚C and pH 5. Multicomponent aqueous solutions containing Cs+, Pb2+, Cu2+, Cd2+, Ni2+ and Zn2+ were chiefly prepared from analytical grade standard solutions (Nakarai Teque, Japan) in the range of 50 to 500 mg/L. The initial pH of the heavy metal solution was controlled to pH 5 with adjusting amounts of HCl 0.1 M and NaOH 0.1 M for each adsorption test. Then 0.05 mg of geopolymer powder was added in 40 ml of the multicomponent aqueous solution of metal ions. After adsorption batch, the supernatant liquid was separated by centrifugation at 100 rpm. The changes in the metal ion contents in the supernatant were analyzed by atomic absorption spectroscopy (AA- 6300 SHIMADZU). The changes in metal ion concentration of the solution were represented as the removal of metal ion by geopolymer adsorption, according to following equation.
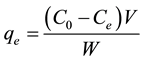 (1)
(1)
where C0 and Ce are the initial and equilibrium concentrations (ppm), respectively, of the metal ion in the solution, V is the volume (L), and W is the weight (mg) of the adsorbent.
3. Results and Discussions
3.1. Properties of Geopolymer Adsorbents
Table 2 shows the surface area (BET), zeta potential at pH 5 and bulk density of the metakaolin based geopo-
![]()
Table 2. Composition and condition of geopolymer synthesis*.
*The curing temperature for the condentation was carried out at 80˚C for 12 hours and thend cured matrix was again heated at 200˚C for 6 hours.
lymers. By using the chemical composition by XRF data we estimated Si/Al ratio in the geopolymers after the polycondensation reaction and it was observed that the values of the ratio were close to the calculated in the beginning. This meant that the polycondensation of MK and silica fume was performed to be geopolymer framework under the described conditions. SEM pictures are shown in Figure 2, the resultant geopolymers with a ratio Si/Al > 4 were condensed like a dense matrix as seen in Figure 2(d) and Figure 2(e). The morphology suggested that the grain size of the geopolymers was decreased with increasing the amount of silica in the framework. Correspondingly, the surface area BET was higher in the GP-1 than those of others due to the high Al content in the geopolymer. Also, an increase of the bulk density was observed, leading the decreasing of the porosity in the samples.
FTIR spectra are shown in Figure 3. The spectral data of MK and silica fume used as raw materials were included. The spectral differences in the FTIR results were found in the low-wavenumber region between 800 to 400 cm−1 and the middle-wavenumber region between 1250 to 800 cm−1. In the low-wavenumber region, the spectrum of silica fume had characteristic the bands at 469 cm−1 assigned to Si-O tetrahedral bending vibration [24] and the MK band at 461 cm−1 and 812 cm−1 assigned to tetrahedral bending mode of T-O (T = Si or Al) and bending mode of Si-O-Al [25] - [27] , respectively. After geopolymerization, both intensities of these two bands decreased and the new band appeared at about 710 cm−1. The spectral data indicated that the formation of tetrahedral Al [Al-O4] was found in the resultant geopolymers [27] . The small band appearing around 1400 cm−1 was related to the asymmetric stretching of the O-C-O bonds of CO32− due to atmospheric carbonation on the surface of powdered products [28] . The absorption bands at 1650 cm−1 were for H-OH vibration, corresponding to the presence of water in the geopolymer [25] [26] . Moreover, the small band centered about 600 cm−1 was caused by T-O-Si symmetric stretching vibrations [29] [30] . In the mid-wavenumber region, a shift in the broad band position for Si-O-Si of silica fume at 1099 cm−1 and Si-O-T from MK at 1084 cm−1 was seen toward 1000 cm−1. Both assigned asymmetric stretching peaks were seen in the geopolymer as assigned to Si-O-Al of geopolymer vibration at 1000 cm−1 [31] - [33] . This band shift suggested strongly formation of geopolymers [34] [35] . Especially, at higher Si/Al ratio, the Si-O-Si band at 1099 cm−1 was observed in the broad band as shoulder.
Furthermore, the XRD pattern in Figure 4 supported the amorphous features of the geopolymers. As seen, the silica fume and MK used had broad peak centered at 22˚ in the XRD patterns. The presence of the sharp peak at 26˚ implied that the MK contained crystalline SiO2 and mica components. After the alkali activation of the starting materials, the scattering diffraction of the broad peak was shifted from ~22˚ to ~28˚ in 2q. This was evidence of the change in the local bonding environment due to the arrangement of the structure during the polycondensation process.
Table 2 contains the zeta potential obtained from the geopolymers at pH of 5. The values of zeta potential are shown in Figure 5. It was possible to observe that the zeta potential decreased from −20 mV to −29 mV when the Si/Al ratio was increased from 1 to 5, respectively, at pH 5. This meant that the increment in the silica content in the framework caused negatively changed groups of O-Si-O− in the geopolymer [36] [37] for the corresponding surface charges expressed. As shown in Figure 5, the values of zeta potential increased, when NaCl concentration increased at 5 wt% and 10 wt%. The zeta potential increased with the increment of SiO2 and a constant value was obtained after the ratio of Si/Al = 2, for the three NaCl concentrations. In other hand, it was observed the zeta potential decrease with the increment of concentration of NaCl of 5% and 10% due to the competitive adsorption of H+ with Cl− for the binding sites of geopolymers at pH 5. For example at Si/Al = 2, each value of
![]()
Figure 2. SEM micrographs of the metakaolin based geopolymers: a) GP-1; b) GP-2; c) GP-3; d) GP-4 and e) GP-5.
![]()
Figure 3. FTIR spectra of raw material and synthesized metakaolin based geopolymers.
![]()
Figure 4. XRD results of raw material and metakaolin based geopolymers.
![]()
Figure 5. Zeta potential in function of the ratio of Si/Al of metakaolin based geopolymer with (®) 0 wt% NaCl, (¡) 5 wt% NaCl and (p) 10 wt% NaCl at pH 5.
zeta potential was changed from −29 mV, −22 mV and −15 mV with a [NaCl] = 0, 5 and 10 wt % concentration, respectively. This behavior indicated that the negatively charge was electro-statically shielded by the added salt.
3.2. Adsorption Studies of Metakaolin-Based
The amount of silica fume in the metakaolin based geopolymers adsorbents has a strong influence on the ad-sorption process. Figure 6 shows the analysis of Langmuir isotherms for single component of metal ion (Figure 6(a)) and multicomponent solution (Figure 6(b)) of metal ions of Pb2+, Cu2+, Cd2+, Ni2+, Zn2+ and Cs+ adsorbed by geopolymers. For Figure 6(a) was observed that the adsorption by GP-2 for Pb2+, Cu2+ and Cs+ predominated to comparison of Cd2+, Ni2+ and Zn2+ which were less than the former ion group. At pH 5, Pb2+ could be present as Pb(OH)+, Pb2(OH)3+, Pb3(OH)42+ and Pb4(OH)44+ but just in a small amounts [38] . However, the experiments of adsorption for Pb2+ could not be performed beyond pH 6.0 due to the low solubility of Pb2+ hydroxide [39] in water, because the lead component is formed as white precipitation at that pH. Therefore, the adsorption test was carried out at pH 5. In contrast, the multicomponent system Figure 6(b) showed that the removal of Pb2+ and Cs+ increased considerately, especially for Cs+ ion. This comparison between Figure 6(a) and Figure 6(b) meant that the geopolymer had selective binding for Cs+. As a result, the adsorption selectivity of GP-2 for a mixture of metal ions was in the following order Cs+ > Pb2+ > Cu2+ > Zn2+ > Ni2+ > Cd2+.
Figure 7 shows adsorption behavior of Pb2+ for several geopolymers at different Si/Al ratio. It was noted that the GP-2 presented better adsorption for Pb2+, in comparison with the GP-1 which containing higher Al amount in the framework, so that the adsorption for Pb2+ ions decreases. It is very interesting to study salt effect of adsorption behavior of the geopolymers. As shown in the results of zeta potential, negative value supported the presence of negatively electrostatic force on the geopolymers. In Table 3 for GP-2, the effect of the addition of
![]() (a)
(a)![]() (b)
(b)
Figure 6. Langmuir isotherms for the adsorption of a) individual metal ions solution and b) mixture of multicomponent of metal ions by GP-2.
![]()
Figure 7. Langmuir isotherms for the adsorption of Pb2+ by metakaolin based adsorbents a pH 5.
![]()
Table 3. NaCl addition to multi-component solution for the adsorption capacity of heavy metal by GP-2.
NaCl was tested with a concentration of 5 wt% and 10 wt% on the m Table 3 mixture solution of metal ions. It is worth noting that the salt effect does not affect considerably in the adsorption of metal ions. For example, Cs+ was adsorbed on the geopolymer with qm = 43, 42 and 43 mg/g for [NaCl] = 0 wt%, 5 wt% and 10 wt%, respectively. Similarly, insignificant changes were observed for the maximum adsorption by GP-2 of Pb2+ and the other metal ions with the increment of NaCl concentration as shows in Figure 8. As seen, the metal ions adsorption by geopolymer at pH 5 was indicated that sodium concentration had no effect on the adsorption of the heavy metal ions. This meant that the heavy metal ions captured by geopolymer might be due to the less effect of electrostatic mechanism. Langmuir equation with Sacatchard analisys was used for geopolymer adsorbents in modeling of the isotherm data for the multi-component solution containing Cs+, Pb2+, Cu2+, Cd2+, Ni2+ and Zn2+. The experimental isotherms are useful for describing adsorption capacity to facilitate evaluation of the feasibility of this process. The isotherm measures the relation between the equilibrium concentration of the adsorbate in the solid phase qe [mg/g] and the equilibrium concentration in the aqueous phase Ce (mg/L) [40] . As known, various mathematical transformations of the classical Langmuir equation, qe = (qmKbCe)/(1 + KbCe), are presented and then, the analysis having transformations of Ce/qe versus qe, providing various useful graphical demonstration manners [41] [42] . The Scatchard transformation can give more compact information about affinity phenomena of sorbent toward analyte. In the present study, the experimental data was applied to the Scatchard transformation was represented by the following equation [43] .
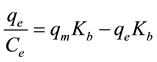 (2)
(2)
where qm is theoretical maximum sorption capacity of sorbent for target solute to form a complete monolayer, and Kb is the constant related to affinity between sorbent and sorbate.
The parameters calculated from the Scatchard plots are collectively listed in Table 4. As seen, Langmuir isotherm model was useful for characterization of specific bindings, because it mainly deal with sorption on specific binding sites. The Scatchard plot analysis for GP-2 is shown in Figure 9. The Scatchard plot is widely used technique in evaluating the affinities of binding sites taking role in a particular adsorption process. As can be seen, the deviation tendency in the plot from the linearity portion resulted in two independent sets in the data. This phenomenon indicated presence of at least two types of binding sites having different affinities toward the metal ions in the geopolymer system. So, the geopolymer adsorbent has high-affinity and low-affinity sites for Cs+ and Pb2+ ions. Hence, the observed linear data were believed to be separately related to different specific bindings. The separately calculated isotherm parameters are tabulated in Table 4. Here, two different specific binding types of metal ions on the geopolymer were found to be observable at pH 5 as designated with H and L, representing high and low affinity. It can be suggested that the specific binding sites of Cs+ and Pb2+ were contained mainly in the geopolymer. This information can be useful in design of novel separation techniques based on adsorption process for selective removal metal ions from waste water. Therefore, furthermore, research would be on the progress on the clear explanation of the adsorption behavior. Additionally, it was interesting to note that in Figure 6 the high binding Pb2+ ion was observed in the single ion system (Figure 6(a)), but the order in Pb2+ and Cs+ was changed for the multicomponent system (Figure 6(b)). This meant that predominant adsorption to Cs+ was occurred in the geopolymer adsorbent, when Pb2+ and Cs+ were competed in the adsorption. As mentioned, the Scatchard analysis of qm and Kb were supported the strong Cs+ binding to the geopolymers.
![]()
Figure 8. Maximum adsorption capacity (qm) of heavy metal ions by GP-2 with different concentration of NaCl. (¡) 0 wt% NaCl, (¡) 5 wt% NaCl and (□) 10 wt % NaCl at pH 5.
![]()
Figure 9. The Scatchard plot analysis for GP-2 at pH 5.
![]()
Table 4. Isotherm parameters of Scatchard plot.
The “H” and “L” symbols represent particular parameters for high- and low-affinity bindings, respectively.
4. Conclusion
On the ability of metakaolin-based geopolymers, the removal of Cs+ and Pb2+ with heavy ion mixture of Cu2+, Cd2+, Ni2+ and Zn2+ was conducted from aqueous solution of mixed heavy metals. The adsorbent behavior was examined as function of the Si/Al ratio in the geopolymer matrix and optimized at Si/Al = 2. The geopolymer worked well for the separation of Pb2+ and Cs+ on the mixture solution of metal ions. The value of the adsorption capacity increased in the following order: Cs+ > Pb2+ > Cu2+ > Cd2+ > Ni2+ > Zn2+ for the mixture of the multicomponent system, while the individual experiment showed higher adsorption in Pb2+ relative to Cs+. Langmuir adsorption model was used for analyzing the efficiency of adsorption of the metal ions onto geopolymers. This suggested that the geopolymer adsorbents have a high selectivity for Cs+ ion. Salt effect on the adsorption behavior indicated that the selectivity was due to the electrostatic force of charged sites of the geopolymers. For evaluating the type of interactions between metal ions and metakaolin-based geopolymer, further research would be in progress in near future.