1. Introduction
Whiteness of the skin is considered as important cultures element in constructing female beauty worldwide [1] particularly in parts of sub-Saharan Africa [2] , where the culture of bleaching has become common among black Africans [3] .
Skin lightening creams alter the chemical structure of the skin by inhibiting the synthesis of melanin and are regulated as drugs not cosmetics [3] .
The active ingredients used are hydroquinone [4] [5] , highly potent corticosteroids and mercury salts [2] .
Over the counter (OTC) skin lightening creams containing hydroquinone are inadequately labeled and exceed the permitted concentration limit of 2% [6] where complications were reported such as skin disorders [2] , and general systemic complications, such as nephritis or neurological disorders secondary to mercurial’s [2] -[7] .
Pregnancy is a period of high risk for the use of skin lightening products, especially during the third trimester [2] , so exposure of placental cells to mercury causes accumulation of the metal in the placental membrane and lowers the membrane fluidity which affects membrane function and causes damage to the developing fetus [6] -[8] .
In Mali, the cosmetics used were either hydroquinone-containing products or mercuric derivatives [9] . In Senegal the danger of corticoids and hydroquinone-based products was reported [2] -[10] .
Chan (2011) [11] , reported the worldwide studies about the lightening creams containing mercury, including seven African’s studies distributed as: Kenya (1972, 1973), South Africa (1974, 1976), Uganda (1974), Malawi (1977) and Senegal (1981).
Although the worldwide awareness regards the danger of this bad habit, there is a lack of information in Sudan, and this study was set out to evaluate the use of cosmetics whitening creams containing hydroquinone, mercury and corticosteroids among the young students in central Sudan.
2. Methodology
2.1. Study Design
A random sample of (16.2%) of the study population, where, students of 19 girls schools from governmental higher secondary school in Wad Medani locality, capital of central Sudan, were selected during January 2010.
1500 well-structured, in-depth and open-ended questionnaire were distributed to 13 academic, 4 co-academic, one commercial and one technical school. The questionnaire distributed randomly to 20% from the total number of students in each school, and then a total of 1187 questionnaire was retained back and evaluated.
All sample members were inclusion to the study after obtaining full permission from relevant competent authorities.
2.2. Statistical Analysis
Statistical evaluations were performed for the collected data and analyzed by means of the chi-square test and Fisher’s exact test to compare the mean differences for various results. The differences were considered to be significant at p ≤ 0.05.
3. Results
Only 1187 questionnaires from the distributed (1500) were returned by students.
Our survey revealed that different whitening creams available at different selling gates in Sudan, ranging from small shop to modern pharmacies, these products contains significant concentrations of the worldwide banned serious chemicals, preliminary information about these cosmetics were presented in Table 1.
The participants distributed between first, second and third class (Table 2). Generally, higher rate of missed values were reported among participants.
Abundance populations (79.6%) were observed in academic school rather than others, whilst high average age was reported among the Co-academic school, more over higher percentages were accumulated at the third class level, Table 2.
The prevalence of the use of these creams was relatively high with low side effects reported among the whole groups.
Cosmetics that contain mercury compounds showed greater abundance among the other ones used by the central Sudan students with similar percentage in different classes, which can be seen clearly in Table 2, besides, a mixture of both Corticosteroids and mercury were ranked secondly, whilst the mixture of the three compounds comes later.
The continuous use of cosmetics was highly rated during the 1st and 2nd years of starting application, particularly within the 1st year, whilst chronic daily administration was observed, Table 3.
The face, as body application area, represents the main body area targeted for these compounds by the students under investigations (Figure 1(b)).

Table 1. Some products (trade name) that used by participant girls, categorized based on the presence of the chemicals under investigations with their content, origin and the local supplying source.
The exact steroid in the product was, a) Clobetasol Propionat, b) Betamethasone, c) Triamcinolone Acetonide, d) Fluocinolone Acetonide. The concentration of * was obtained from Al-Saleh et al. (1997) [12] , while for ** from Al-Ashban et al. [6] .

Table 2. Characterization of the study population (n = 1187).
Socio-economic patterns that presented in Table 4, showed that the major supply source where the participants brought these compounds was the local small shop with noticeable records for the modern pharmacies.
Although the majority of participant believes that these cosmetics were economically expensive, they insist to buy them even through their own saving financial sources, whilst mother acts as a main supporter, Table 4.
On the other hands, the use of cosmetics was a personal habit for self satisfaction, which can be seen clearly by the infection source data, besides direct colleague imitation had a considerable impact on this attitude, see Table 4.

Table 3. Patterns of use of skin lightening cosmetics (n = 1187).
 (a)
(a)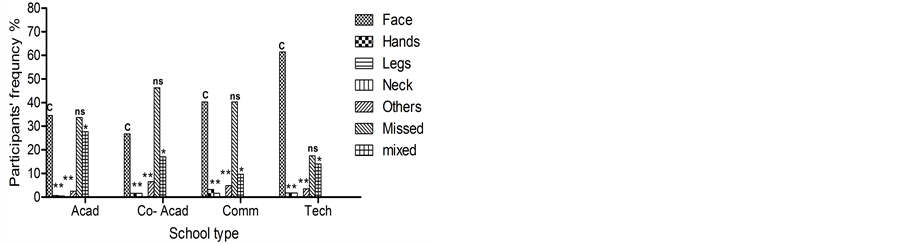 (b)
(b)
Figure 1. Participant’s cosmetics use, (a) The percentage of the purpose of the use among the different school participants; whilst (b) The percentage of the area of application on the participant body. Data presented as frequency (%) whilst, *p < 0.05, **p < 0.01 and ***p < 0.001, ns: not significant statistically.

Table 4. Socio-economic patterns of cosmetics use (n = 1187).
The use of these agents for cosmetics reasons were significantly shown in comparable to other purposes regardless the high percentage of missed value reported among the participants.
In spite, the high awareness that appeared by the whole participants regard the serious side effects of use, there was a significance lack of doctor contact, with huge missed data, Figure 2.
4. Discussion
Although, the whitening products containing corticosteroids, hydroquinone and mercury have been banned worldwide in cosmetics and soaps [2] , these chemicals still appear on packaging materials in cosmetics that imported and available in Sudan local market.
The higher secondary school students of central Sudan represented by the Wad madani locality were our selected population sample, this due to that they have suitable ages with intermediate education and awareness, besides Wad madani as a city represents the whole central Sudan area with huge people diversity.
The university students were not targeted, because the majority of them were already use these products (self communications), also we thought that this phenomenon must be with less spreading among the students of
 (a)
(a) (b)
(b)
Figure 2. Participant’s awareness regard the hazards of the cosmetics use, (a) The percentage of the reported side effects among the different school participants; whilst (b) The percentage of the participant doctor contact regard the side effect control. Data presented as frequency (%) whilst, *p < 0.05, **p < 0.01 and ***p < 0.001.
higher secondary school.
Our survey initially rang the bell to all authorities, because these products can be easily bought not only from the small shops, but also from the modern pharmacies that responsible for the health protection and maintenance. Besides, to reflects the drawback of mass media and the fashion industry which play important roles in reinforcing the yearning for white skin, because advertisements also play important roles in shaping ideal self images for consumers [1] .
The high frequency rate of the missed data in this survey was attributed to that, the questionnaires distribution was done indirectly through the teachers of schools and not by investigators, But we believe that these missed values will not affect the final findings, because the sample size was very big and the collected data was more than 10% of the population and fall within the statistically accepted margin.
Normally, in Sudan the majority of the student were belong to the academic school, so less average age were reported, whilst the co-academic school student differ significantly among all participants, besides the girls school at the target area were mainly academic with few other school type, moreover, the non-academic schools were newly constructed as seen by the missed data for 3rd class.
Our findings revealed that, there was a high prevalence of bleaching cosmetics use among the participants with less reported side effects, this may be attributed to, they were only at starting point of use, also their youngest age can resist to some extent the side effects, whilst the participants may afraid for talking about these due to personal reasons.
The use of cosmetics that contains mercury compounds was significantly high; this may be due to the affordability, whilst literature reported more danger effect for mercury, because short duration of use will lead to serious side effects.
Participants decline the use of these creams after 2nd year of use which can explain by, in the 3rd year the academic pressure was very high due to the stopping exams for university entrance, also may be due to the appearance of some side effects and the awareness increase among the students.
It is obviously, seen that the main body area for application was the face, this due to that the face was the place of beauty and attraction in female, also face has relatively small surface area, but the girls thought that the white face is more beautiful than the black one regardless the homogeneity with other body parts which was defiantly incorrect. Moreover beauty will never be more important than the health state in mature well-educated people. On the other hands and due to religious reasons, few parts of the body were uncovered including face.
As the study was carried out at central Sudan where the majority of the populations were related to the Arab race origin whom had relatively white skin color, the rate of applying of the danger chemicals was very high as a direct need for the new look.
To get these banned products from modern pharmacies was unacceptable matter; because pharmacist was the most health profession aware about the hazardous of these products, thus ethically he must be a main barrier against the spread of this phenomenon.
Socio-economic findings revealed that, the majority of participants believes that these cosmetics were economically expensive, but they insist to buy them even through their own saving financial sources, whilst mother acts as a main supporter, because in the past their mother do similar things and believe that these will increases their chance in getting marriage. This was in line with Hamed et al. (2010) [13] , who mentioned that people who use skin lightening products are more likely to believe that lighter skin tone has a positive role to play in self esteem, perception of beauty and youth, marriage, and employment opportunities compared with non-users [13] .
Participants not only imitated their colleagues in using these things, but also use it for self satisfaction purposes; this may be due to the media effect or lack of enough knowledge. On other hands, normally, participants use these products for cosmetics reasons only; few use them for curative needs.
In spite, the high awareness that appeared by the whole participants regard the serious side effects of use, there was a significance lack of doctor contact among the students, and these may be due to the financial problems or the health ignorance. Normally, they did not contact the doctors unless they exhibited serious complications due to the repeated use of their cosmetics from supplier whom they more trusted in.
5. Conclusions and Recommendations
The importance of this study arises from its aims that set out to minimize the risk factors of these whitening creams depending on counseling the community.
43% of the participants using bleaching creams contain steroids, mercury and hydroquinone and only 10.6% using cosmetics do not contain these compounds while 46.4% were missed, and these creams were imported from outside. 51.6% of these young girls had used cosmetics to lighten their skin for between one to three years and 3.3% claimed that they had some irritations.
The presence of these chemicals, mercury, hydroquinone and corticosteroids, which had already been banned worldwide in cosmetics and soaps must be viewed with serious concern, especially when most of the users claimed that they were ignorant of the health implications involved.
On the other hand, corticosteroids must be restricted for prescription (POM) and not sailed as OTC drugs.
Finally we recommended the following suggestions: counseling lectures that cover all higher secondary school girls in Wad madani locality, to discuss the hazard of these toxic substances in these whiting creams, whilst the important role of the related authorities must be activated.
Post marketing surveillance to withdraw unlicensed products and counseling by other safe alternative products, besides, the corticosteroid must be restricted for prescription only (POM).
Practical laboratory study to check the active ingredients and their concentration to these products were highly appreciated as soon as possible. Also checking every batch of these products that imported legally from the national Lab, moreover, the industries and imported cosmetics companies, must state all the concentrations, percentage of the all ingredients.
Epidemiological surveillance is required to withdraw all the cosmetics that contain corticosteroid, mercury and hydroquinone from the market, at the same time mass media counseling and activating the direct family role in counseling the younger girls.
Acknowledgements
We grateful to Ministry of Health, Ministry of Education, Drug Information Centre and Drug Revolving Found, Gezira State, Sudan, for all support and facilities.