Stress Degradation Studies on Lisinopril Dihydrate Using Modified Reverse Phase High Performance Liquid Chromatography ()
1. Introduction
Lisinopril is chemically described as l-proline, 1-[N2-(1-carboxy-3-phenylpropyl)-l-lysyl]-, dihydrate or (2s)-1- (2s)-6-Amino-2-[[(1s)-1-carboxy-3-phenylpropyl] amino] hexanoyl] pyrrodine-2-carboxylic acid dihydrate. Its empirical formula is C21H31N3O5∙2H2O. It is a white or almost white, crystalline powder with a molecular weight of 441.52. It’s soluble in water, sparingly soluble in methanol; practically insoluble in acetone and in anhydrous ethanol [1] [2] .
Lisinopril is used to treat high blood pressure (hypertension), congestive heart failure, and to improve survival after a heart attack. Lisinopril works by causing blood vessels to relax, lowering blood pressure and increasing the supply of blood and oxygen to the heart. For this reason it is also sometimes used, alongside other medicines, to treat circulatory problems associated with diabetes [3] -[5] .
Drug stability plays a very important role in pharmaceutical research and development for a newly developed drug product, stability analysis not only provides useful information regarding the degradation of the drug product, but also determines an expiration dating period of the drug product.
For the purpose of safety and quality assurance, most regulatory agencies such as FDA require that an expiration dating period be indicated on the immediate container label for every drug product on the market [6] .
Stability testing is the primary tool used to assess expiration dating and storage conditions for pharmaceutical products. Many protocols have been used for stability testing, but most in the industry are now standardized on the recommendations of the International Conference on Harmonization [7] -[9] . These guidelines were developed as a cooperative effort between regulatory agencies and industry officials from Europe, Japan, and the United States.
Degradation reactions in pharmaceutical formulations take place at definite rates and are chemical in nature. They depend on such conditions as concentration of reactants, temperature, pH, radiation, and catalysts.
The common stress conditions include acidic pH, basic pH, neutral pH, different temperature and humidity conditions, oxidation, reduction and photo-degradation [8] . These studies help to determine the significantly related substances to be used in method development, and to determine the degraded product formed under stress conditions [10] .
The present study aimed at investigation of the photo-thermal stability of lisinopril dihydrate.
2. Materials and Methods
2.1. Chemicals and Reagents
All chemicals and reagents used were of a HPLC grade. Lisinopril dihydrate was kindly supplied from Zhejiang Huahai Pharmaceutical CO. Ltd. (Taizhou, China). Tetra-n-butyl ammonium hydroxide 40% aqueous solution was obtained from AppliChem, Germany. Acetonitrile HPLC grade was obtained from BDH Labs, England. Orthophospharic acid 85% were obtained from BDH laboratory, England.
2.2. Equipment
1) HPLC was performed using a Waters Breeze 2 HPLC System consisting of LC 1525 binary pump, 2489 UV/Visible Detector, and 2707 auto sampler.
2) Column used was C18, Waters Spherisorb® 5.0 µm ODS2 4.6 mm × 250 mm.
3) Sartorius model cp224s balance.
4) Mi 180 Bench pH meter, MARTINI instruments.
5) Ultra violet radiation (UV Lamp λ254 - λ265) with Obligatory eye protection-Model-M014492 BDH-England.
2.3. Preparation of Mobile Phase
Mix a solution of 0.03 M Tetra butyl ammonium hydroxide (Solution was prepared by diluted 20 ml of tetra butyl ammonium hydroxide 40% aqueous solution to 800 ml with distilled water and adjusted to pH 6.5 with 10% of aqueous orthophosphoric acid and diluted up to 1000 ml with distilled water) and acetonitrile with a ratio of 3:1 respectively and degassed.
2.4. Preparation of Standard Stock Solutions
Stock standard solution having concentration of 0.1 mg/ml was prepared by dissolving pure drug of lisinopril dihydrate in distilled water.
2.5. Preparation of Solution for Calibration Curve
Standard stock solution was 0.1 mg/ml; these solutions were further diluted to get solutions of concentrations 10, 20, 30, 40, and 50 µg/ml of lisinopril dihydrate.
2.6. Preparation of Lisinopril Dihydrate Sun Decomposed Product
Few grams of lisinopril dihydrate solid were placed between two glass plates (20 × 20 cm), sealed with gum tape and directly exposed to sunlight for six months (March to August). Samples were taken every month and tested for degradation by HPLC.
30 mg of lisinopril dihydrate sun decomposed was dissolved in 100 ml water and analyzed by RP-HPLC.
30 mg of lisinopril dihydrate was weight and dissolved in 100 ml water. The solutions were exposed directly to sun light. Five ml of samples were taken at 30, 60, 90, 120, 150 - 300 minutes, transferred to 25 ml volumetric flask and the volume was completed to the mark with the distilled water and analyzed by RP-HPLC
2.7. Preparation of Lisinopril Dihydrate UV Decomposed Product
30 µg/ml of lisinopril dihydrate solution was prepared and transferred to a stoppered tube. The solutions were placed under UV radiation at λ254 nm. Five ml of samples were taken at 30, 60, 90, 120, 150 - 300 minutes, transferred to 25 ml volumetric flask and the volume was completed to the mark with the distilled water.
2.8. Preparation of Lisinopril Dihydrate Buffering Thermal Decomposed Product
Serial buffer solutions with pH 2, 4, 6, 8, 10, and 12 were prepared. 25 ml of lisinopril dihydrate aliquots (30 mg/100 ml water) were pipette, and separately transferred to six 100-ml volumetric flask and each volume was completed to 100-ml with one of the universal buffer solution mentioned above. Each sample was put in water path thermo stated at 70˚C. Ten mls of each sample were taken at 0, 30, 60, 90, 120, 300 minutes and transferred separately to a small beaker and cooled with iced water; 5 ml from these solutions were transferred to 25 ml volumetric flasks, and the volume was completed to the mark with distilled water (Table 1) and analyzed by RP-HPLC.
2.9. Preparation of Lisinopril Dihydrate with Pharmaceutical Excipients Sun, UV, and Thermal Decomposed Product
The required pharmaceutical excipient was weighted (Table 2), transferred to a 100 ml volumetric flask and dissolved with small volume of distilled water. Twenty-five ml of lisinopril dihydrate (30 mg/100 ml-water) was added to the flask containing the pharmaceutical excipients; the volume was completed to the mark with distilled water. The flask was exposed directly to sunrays. Samples of 10-mls were taken at 15, 30, 45, 60, 75, 90, and 105 and 120 minutes, each sample was separately transferred into 25 ml volumetric flask and diluted to the volume with distilled water (Table 2) and analyzed by RP-HPLC. The some preparations were repeated under UV light, sun light and in a thermostated water path at 70˚C.
3. Results and Discussion
HPLC method was modified for the analysis of lisinopril dihydrate using the mobile phase mentioned above with a flow rate 1.0 ml/min, wave length detection at 210 nm on a C18 column (waters 250 × 4.6 mm, 5 µm) (Table 3), revealed good resolution and peak shape for lisinopril dihydrate (Figure 1).
Modified HPLC method was found satisfactory for analysis of lisinopril dihydrate and degraded products. System suitability parameters were used to the analysis lisinopril dihydrate sun, UV, and thermal decomposed.
For quantitative determination of lisinopril dihydrate, the calibration curve was plotted for the concentration range 10 - 50 µg/ml. Calibration curve plots were constructed using five standard solutions of different concentration .The statistical parameters and linear regression equation calculated from the calibration curve is given in Table 4. The linear regression (r2) was 0.9994 (Figure 1 and Figure 2). Limit of Detection (LOD) was calculated by using formula 3.3 (σ/S) where σ is standard deviation of the response and S is slope of the calibration curve [11] -[13] . LOD was found to be 0.61 µg/ml. Limit of Quantitation (LOQ) was calculated by using formula 10(σ/S) [11] -[13] was found to be 1.87 µg/ml.
With the above selected method parameters, system suitability testing provided adequate good resolution for analysis to be performed for the resolution of lisinopril dihydrate and its degradation products. The stability of the lisinopril dihydrate solid was tested exposed directly to sun light for six months, sample was tested every month by HPLC (Table 5). It’s was found that, lisinopril dihydrate solid were slightly affected by sunlight in a way dependent upon time of expose to sunlight,

Table 1. The effect of pH-value on the reaction rate of lisinopril dihydrate thermal-decomposed.

Table 2. The effect of some pharmaceutical excipients on the stability of lisinopril dihydrate sun decomposed.

Table 3. Conditions used for chromatography analysis.

Table 4. Results of standard calibration curve for the analysis of Lisinopril dihydrate by HPLC (r2 = 0.9994).
LOD = 0.61 µg/ml, LOQ = 1.87 µg/ml.

Table 5. The effect of time on the reaction rate of lisinopril dihydrate solid sun-decomposed.

Figure 1. Test HPLC chromatogram for the analysis of different concentrations of lisinopril dihydrate reference to illustrate calibration curve of method.
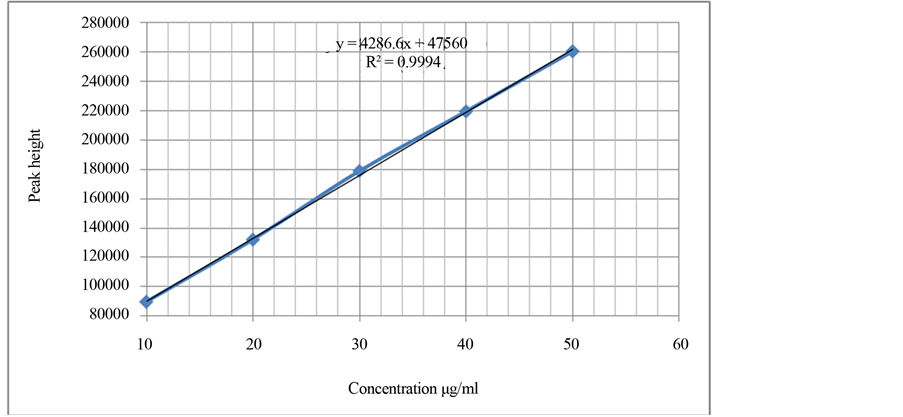
Figure 2. Amount injected versus peak height of Lisinopril dihydrate standard to demonstrate calibration curve (linearity).
Lisinopril dihydrate sun-decomposed reveals that it lost about 10% of its initial concentration when directly exposed to sun light over period of six month (March to August). The reaction rate of lisinopril-2H2O sun-decomposed was found to be increase with the increase of time of exposure to sun light. It was clear that lisinopril-2H2O in aqueous media was rapidly degraded when compared with that in solid forms Table 6. It is a straight forward observation, as it is a well known fact that chemistry in the aqueous state is more rapid reaction than that in the solid form [14] . The stability of the lisinopril dihydrate solution UV decomposed was tested by placed sample under UV light at 254 nm for interval times. The UV sample was analysis using RP-HPLC. The results of analyzed UV decomposed products were presented are shown in Table 7. The effect of UV light on the stability of lisinopril-2H2O solution was studied. The drug was found to be less stable than affected of sun light (Table 6).
The reaction rate of thermal decomposition was increased with the increase in temperature (Table 8). Lisinopril dihydrate was thermally unstable with increase pH value (Table 1). In this work, the effect of some pharmaceutical excipients on the stability of lisinopril-2H2O Sun, UV, and thermal decomposed were studied, some excipients were found to decrease the reaction rate as talcum, dibasic calcium phosphate (dibasic Ca.ph), and magnesium stearate (Mg.S), and other were increase the reaction rate such as a vicel and Maize starch (Table 2) by sun, UV, and thermal effected respectively.

Table 6. The effect of time on the stability of lisinopril dihydrate solution sun-decomposed.

Table 7. The effect of time on the stability of lisinopril dihydrate solution UV Light decomposed.

Table 8. The effect of temperature on the reaction rate of lisinopril dihydrate solution.
4. Conclusions
It can be concluded from this work that lisinopril dihydrate is found to be stable under sun light and UV light into solid and liquid forms. The HPLC method was found to be suitable for the study of kinetic of photo-thermal decomposition of lisinopril and its quantitative determination in the presence of photo-thermal decomposition products.
Some pharmaceutical excipients were found to enhance the stability of cefixime trihydrate; therefore it’s recommended that such excipients be used in the formulation.