Restoration-Focused Germination and Development of Five Central Mexican Oak Species ()
1. Introduction
In Mexico, oaks (Quercus spp.) are widely distributed in temperate forests, forming pure stands or associations with conifers or other species (Challenger, 1998; Valencia-Avalos, 2004). Oak forests occupy 9 × 106 ha of Mexican land and are located mainly in the mountainous regions of the country (Challenger, 1998). Between 127,000 and 167,000 ha of temperate forests are lost every year (Masera et al., 1999), affecting many areas of forests occupied by these species (Alfonso-Corrado et al., 2007). Deforestation of large areas of land has profound ecological and environmental effects (Greipsson, 2011) and entails the reduction or loss of biodiversity and ecosystem function and services (Brown & Lugo, 1994; Esquivel et al., 2008; Chazdon et al., 2009).
Natural land recovery might occur eventually if secondary succession is allowed to prosper without any other disturbance (Lamb & Gilmour, 2003; Greipsson, 2011). However, this process is slow, often obstructed, and its outcomes are uncertain (Brown & Lugo, 1994; del Moral et al., 2007). When forest recovery is not possible by natural processes, human intervention is necessary, either to initiate or accelerate secondary succession. Doing this restores both ecosystem services and biodiversity (Chazdon, 2008; Greipsson, 2011). Several actions have been suggested to restore biodiversity in damaged ecosystems (Lamb & Gilmour, 2003; Shono et al., 2007; Greipsson, 2011). Among these, planting tree species of ecological and economic importance seem to adequately reduce soil erosion, increase biological diversity and accelerate the natural recovery process (Lamb & Gilmour, 2003; Lamb et al., 2005; Vieira & Scariot, 2006). This action is particularly useful under certain conditions, including scenarios where the degraded land is distant from the remnant forest (which acts as a resource for plants and animals), or when seeds are poorly dispersed (Lamb & Gilmour, 2003).
Some authors have suggested the use of native species in restoration programs considering that they are well adapted to the environment and therefore, are more likely to become established (Vázquez-Yañes & Batiz, 1999; Lamb & Gilmour, 2003). In Mexico, reforestation policies have been focused on nonnative species, resulting in failure in the restoration programs (Segura-Burciaga, 2005; Cervantes et al., 2008). Recently, these environmental policies have changed, to promote reforestation using native species (Vázquez-Yañes et al., 1999; Cervantes et al., 2008). Nonetheless, the processes of seed germination and seedling establishment of many oak native species that are useful for restoration of Mexican forests are far from being understood.
Despite the fact that several species of the genus Quercus produce viable acorns with high germination rates, their natural regeneration rate remains low (Zavala-Chávez & García-Moya, 1997). Factors such as desiccation (Zavala-Chávez, 2004), fruit predation and parasitism (Kajimura & Fukomoto, 2005), or irregular acorn production (Kelly & Sork, 2002; Alfonso-Corrado et al., 2007) have been identified as causes of unsuccessful seedling establishment. Acorns are considered as recalcitrant because desiccation occurs in a short period of time after seed maturation (Zavala-Chávez, 2004).
After dispersal, recalcitrant seeds quickly lose their germination capacity due to exposure to low humidity levels (Kermode & Finch-Savage, 2002; Zavala-Chávez, 2004). Daws et al. (2005) have suggested that a relatively large fruit increases germination rate and growth efficiency in recalcitrant species, thus reducing exposure time to predators, or investment of resources for defense. It has been reported that large acorns increase germination, seedling growth and survival rates (Navarro, 2006; Quero et al., 2007; Yi & Yang, 2010).
In this study, observations of populations of five oak species (Quercus eduardii Trel., Q. grisea Liebm., Q. potosina Trel., Q. resinosa Liemb. and Q. syderoxyla Trel.), in the naturally protected area of Sierra Fria, Aguascalientes suggest that natural regeneration by acorns is infrequent. The Sierra Fría forest has been recognised as a priority area for conservation and identified at the regional level as a biodiversity hotspot in central Mexico (SEDESO, 1993). However, from 1930 to 1950, this forest was subject to intense degradation activities including timber extraction and the introduction of agriculture and grazing. This anthropogenic perturbation was responsible for damaging the oak population and also for the severe erosion of the forest’s soils (ChapaBezanilla et al., 2008; Minnich et al., 1994).
Consequently, this study evaluates factors that affect germination of these species and also investigates those implications, in order to recommend low cost seed management actions for restoration purposes. The aims of this study were: 1) to estimate acorn viability; and 2) to evaluate the effect of acorn size on germination, seedling growth and survival rates of these species.
2. Methods
2.1. Study Area
The study was carried out in the protected natural area of Sierra Fria, Aguascalientes, Mexico (Figure 1), which is part of the floristic province of Sierra Madre Occidental that comprises an area of 112,090 ha. The area has a temperate, semi-dry climate; the rainy seasons is in summer, with a mean annual temperature of 14.5˚C, and a mean annual precipitation of 664 mm. Vegetation is dominated by oak forests, or oak-pine forests at altitudes between 1900 and 3050 masl (SEDESO, 1993).
2.2. Study Species
For this study we select the five most abundant oaks species of Sierra Fria: 1) Quercuseduardii Trel. red oak (Lobatae), (trunk height, varying between 5 - 20 m), 2) Q. grisea, Liebm. White oak (Quercus), (trunk height, between 4 - 10 m), 3) Q. potosina Trel. white oak (Quercus), (trunk height, varying between 3 - 15 m), 4) Q. resinosaTrel. white oak (Quercus), (trunk height, varying between 6 - 10 m), and 5) Q. sideroxyla Trel. red oak (Lobatae), (trunk heights varying between 5 and 30 m) (Table 1).
2.3. Data Collection
During fruit production (August-November 2005), thirty-five individuals of each species were selected and approximately 100 to 200 acorns were collected directly from each individual, thus reducing seed damage from insects or other factors (Zavala-Chávez, 2004). A total number of 25,000 acorns (5000 of each species) were placed in plastic bags and taken to the laboratory for further analyses.

Figure 1. Collection sites of acorns in the Sierra Fría Aguascalientes, Mexico.

Table 1. Life history and ecology characteristics of oaks from Sierra Fría, Aguascalientes, Mexico.
2.4. Acorn Viability
The floating method is economic and was used to identify viable acorns (Gribko & Jones, 1995). All were placed in vessels containing water and those that floated were considered dead (probably due to endosperm destruction by insects or rotting), whereas those that sank immediately were considered as viable. Usually, viable acorns are free from fungi or insects and therefore, the endosperm and embryo are ready to germinate (Hartmann & Kester 1981); acorn infestation was also recorded.
2.5. Acorn Size Classification
Viable acorns of each species were weighed on an analytical scale of 0.001 g (VP213CN Ohaus Corporation, Pine Brook, NJ, USA) then classified according to their fresh weight in three categories (small, medium and large) (Table 2). Acorn size categories were established qualitatively to get similar sample size, for a balanced statistic design.
2.6. Germination
Acorns from each species and size category were completely immersed in tap water for 24 hours. When recovered from the water they were planted in closed plastic boxes containing wet agrolite. The boxes were then placed in a green house at the Autonomous University of Aguascalientes. Acorns were watered weekly and germination was registered every week during three months. An acorn was considered germinated when its radicle was 5 mm long. Germination percentage data were analysed with a Kruskal-Wallis test (Zar, 1984) using the JMP software (SAS Institute Inc., 2001).
2.7. Growth and Survival
Once the radicle was approximately 1.5 cm long, seedlings were placed in individual plastic bags with a mixture of 40% soil, 50% litter and 20% agrolite. Soil and litter were collected from each species collection site and acorns were sown in their corresponding mixture. Growth (shoot height) and survival were recorded weekly during ten months. One-way ANOVA for each species was used to evaluate growth and survival of seedlings from three acorn size classes, using the JMP software (SAS Institute Inc., 2001). Survival data were arcsentransformed to stabilize the variance.
3. Results
Acorn viability varied among species (Figure 2) with Q. sideroxyla showing the lowest viability (25%), and Q. grisea the highest (85%). In the other hand, the lowest infestation by species of the Curculionidae family was

Table 2. Category sizes of acorns according to their weight (small, medium and large) of five species of oaks from Sierra Fría, Aguascalientes, Mexico. Last column shows the average weight (±1 SD) of acorns of each species.
Different lower-case letters indicate significant differences among acorn sizes for each species (P < 0.05).

Figure 2. Acorn viability (%) and infestation by curculionids (%) of five species of Quercus in Sierra Fría, Aguascalientes, Mexico.
found in Q. grisea (15%), whereas the highest was found in Q. potosina (24%) and Q. sideroxyla (19%) (Figure 2). Additionally, acorn weight also varied among Quercus species (Table 2). Acorn size range varied for each species. Mean acorn size category were different among species according to a one-way ANOVA (Table 2). Mean acorn weight of Q. resinosa was significantly higher (P < 0.05) than Q. eduardii.
Germination percentages for acorns of different category sizes in the five species of Quercus are shown in Figure 3. Germination was significantly higher in medium size acorns of Q. sideroxyla, Q. eduardii and Q. potosina (X2 = 15.5; P < 0.05), whereas in Q. resinosa and Q. grisea germination was significantly higher in the largest size class (X2 = 24.95; P < 0.05). However, at species level, Q. grisea showed a significantly higher acorn germination percentage than Q. resinosa (X2 = 4.057; P < 0.05). The other three species showed intermediate germination percentages that also differ from Q. sideroxyla (X2 = 3.47; P < 0.05).
Seedlings of Q. eduardii, Q. potosina and Q. sideroxyla, showed no significant differences in shoot height among small, medium and large acorn category sizes (Figure 4). However, seedlings from the largest acorn sizes of Q. grisea and Q. resinosa were significantly taller than seedlings from medium and small acorn size (F = 49.02; P = 0.002). At species level, seedlings shoot height did not differ among the types.
Finally, our results showed that survival percentage was significantly higher in medium size acorns of Q. sideroxyla, Q. eduardii and Q. potosina (F = 23.45; P = 0.01), whereas in Q. resinosa (F = 25.01; P = 0.001) survival was significantly higher in the largest size class and Q. grisea (F = 24.95; P = 0.001) were significantly higher in the medium and largest size class. However, at species level, Q. resinosa showed a significantly lower survival percentage than the other species, with the exception of Q. eduardii (F = 4.057; P = 0.05) (Figure 5).

Figure 3. Germination (%) in acorn size classes of five species of Quercus in Sierra Fría, Aguascalientes, Mexico, and mean germination (%) of each species. Different lower-case letters indicate significant differences among acorn sizes for each species (P < 0.05). Different capital letters indicate significant differences among species (P < 0.05).

Figure 4. Mean shoot height (x ± 1 SD) of seedling from small, medium and large acorn sizes of five species of Quercus in Sierra Fría, Aguascalientes, Mexico, and mean shoot height for each species. Different lower-case letters indicate significant differences in shoot height among acorn size classes for each species (P < 0.05). Same capital letters indicate no significant differences in shoot height among species (P < 0.05).
4. Discussion
The number and size of seeds produced in the genus Quercus species are important in population level adaptation, since it ensures the establishment and growth of seedlings (Quero et al., 2007; Yi & Yang, 2010). According to Branco et al. (2002), the process of establishing a new seedling must start by evaluating the viability and seed infestation. This is crucial because viable seed selection is fundamental to guarantee the success of conservation programs and the restoration of species with regeneration problems (Martinez et al., 2006).
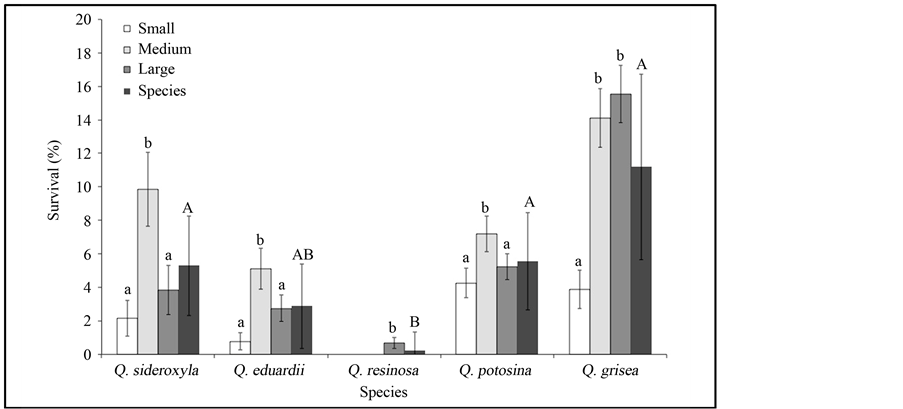
Figure 5. Survival (%) of seedlings from different acorn sizes of five species of Quercus in Sierra Fría, Aguascalientes, Mexico, and mean survival (%) of each species. Different lower-case letters indicate significant differences in survival among acorn size classes for each species (P < 0.05). Same capital letters indicate no significant differences in survival among species (P < 0.05).
Oak species suffer high insect infestation that reduces viability and germination of acorns (Gomez et al., 2003). In our study, the percentages of infestation by beetle larvae (Coleoptera: Curculionoidea) are low (<25%) compared to the averages of over 40% reported in other oak species from America (Thadani & Ashton, 1995; Gomez et al., 2003), Asia (Hou et al., 2010; Yi & Yang, 2010) and Europe (Csóka & Hirka, 2006). There are also different methods for collecting seeds, which can influence the viability of the acorn (Bonner, 2003). In this study the seeds were collected directly from the tree according to Zavala-Chávez (2004), and according to our results this method could be recommend to be employed in subsequent regional studies.
It has been documented that infested seeds with little endosperm damage may germinate in the same way as undamaged acorns and generate healthy seedlings (Gribko et al., 2002; Xiao et al., 2007). It was found that 20 to 30 percent of infested seeds germinated in the five species. However, none of the seedlings survived for more than three months. In this study, we do not recommend the use of infested seeds (those with evidence of small holes or that float in water) in reforestation or restoration. Significant damage to the endosperm and embryo can occur in the infested seed (Fukumoto & Kajimura, 2000; Hou et al., 2010), resulting in vain work efforts and economic resource investment, as well as to obtain negative results when establishing plants.
Seed size is an important feature that influences plant fitness (Harper, 1977; Dalling & Hubbell, 2002), but it may be influenced by the environment (Willan, 1985; Takahashi et al., 2011). We found different evolutionary strategies present in the sexual regeneration of species. Two oak species have sexual reproduction only, Quercus grisea and Q. resinosa, where the large seed size has the highest percentage of germination, survived and grew. Large seeds confer evolutionary advantages by allowing larger cotyledons and rapid movement of its reserves (Branco et al., 2002), increased drought resistance (Takahashi et al., 2011) and increased germination and seedling development (Navarro et al., 2006; Quero et al., 2007). These two species inhabit dry, open forests with little mulch on the ground; hence their seeds are exposed to rapid drying. In semi-xeric forests like Aguascalientes, recalcitrant seed death is usually rapid (Zavala-Chavez, 2004), so the recruitment of large seeds is crucial to successful establishment (Eriksson & Jakobsson 1998; Dalling & Hubbell, 2002).
However, the medium size category with the highest germination, seedling survival and growth were present in Q. eduardii, Q. potosina and Q. sideroxyla. These three species exist in a less arid habitat and have clonal propagation and mast-seeding years (Alfonso-Corrado et al., 2007) unlike Q. grisea and Q. potosina. In oak species that have mast seeding, predators have been shown to preferred large seed (Pons & Breaks, 2007). This finding appears to represent an evolutionary disadvantage for the species, but we have observed that smaller seed size improves resistance to depredation, thus ensuring the successful establishment of seedlings (Gomez, 2004; Yi & Yang, 2010).
A heuristic model developed by Ericksson & Jakobsson (1998) suggests that a medium seed size allows for greater distribution and regional abundance of the species. Q. eduardii and Q. potosina combine vegetative propagation with sexual reproduction and, due to these being the most abundant species in the Sierra Fria (Alfonso-Corrado et al., 2004; Alfonso-Corrado et al., 2007), the higher fitness of their medium-size acorns seem to function efficiently. Additionally, Q. sideroxyla is a species that has specific environmental requirements and a very small niche (Alfonso-Corrado et al., unpublished dates), so it is likely that these same strategies have allowed it to survive. However, future studies are needed to validate this theory.
An understanding of basic biological regeneration at a local scale of five oak species is important because these species present genetic adaptation and local ecology (Alfonso-Corrado et al., 2004, 2007; Rosas-Osorio et al., 2010; Gorgonio-Ramírez, 2012). Choosing the right seed size for reforestation or restoration in disturbed areas of Sierra Fría not only involves the mass production of plants, but also an understanding of their reproductive strategies and the environmental requirements of the species as suggests Vieira & Scariot (2006). Likewise, restoration programs should understand that the fruit size is not a good attribute for comparisons among species because acorn size does not uniformly influence germination percentage, growth or survival among species.
In this work, recommendations for reforestation or restoration programs in the Sierra Fría advocates the collection of large seeds only in Quercusgrisea and Q. resinosa, and large and medium size seeds in Q. eduardii, Q. potosina and Q. sideroxyla. After collection, viable seeds must be selected, germinate them in a greenhouse for six months allowing an adequate growth, then planting a mix of native oak species in the early rainy season facilitates the development of seedlings and may be sufficient for restoration of the degraded forest. This strategy could be appropriate for severely disturbed forests where there usually are altered abiotic parameters, and on which more than 70% of the survival of regenerated plants depend (Clark-Tapia et al., unpublished dates) and ensures the recovery of the Sierra Fria, an area that has been historically subjected to livestock management.
Acknowledgements
We thank to Sierra Fría people and José Medina Flores from Instituto de MedioAmbiente del Estado de Aguascalientes for their logistic support of our fieldwork. Margarita de la Cerda to identify these species. We are also grateful to Tania Sánchez and Francisco Naranjo. Financial support was provided by CONAFORCONACYT (14074).
NOTES
*Corresponding author.