1. Introduction
The central nervous system is the superior organ of organism. It is responsible for vital organic functions and the animal’s perception of the environment. The central nervous system is also the centre of consciousness. Sedation is a mild form of depression of the central nervous system. The animal is a wake, but calm and free of nervous tension. Anaesthesia, on the other hand, is total loss of sensation. It includes depression of the central nervous system, characterized by the loss of sensibility and consciousness [1] [2] .
The mink (Neovison vison) is a small member of the family Mustelidae. It typically has an elongated body shape, short legs and sexual dimorphism. Due to high surface-to-mass ratio, the mink has to sustain higher resting metabolic rate than other animals of the same body size [3] [4] . This sets special demands for its medical treatment in laboratory experiments. Sedation and anaethesia are mainly used for haematological and clinicalchemical examination and short-term surgical procedures in the mink [1] [5] [6] . Any documentation is available on sedation during electrophysiological evaluations in this species.
In our previous electrophysiological study [7] we used medetomidine for sedation in farmed fox (Alopex lagopus). This drug worked well providing ideal sedation for measurements of electroencephalography (EEG), electrocardiography (EEC), and respiration. Medetomidine has been successfully used to induce immobilization in blue foxes, too [8] . In the present study, the aim was to apply medetomidine sedation for electrophysiological measurements in farmed mink before and during euthanasia.
2. Material and Methods
Altogether 15 adult male mink were employed in the present study. Body weights of animals varied between 1307 and 2298 grams. All the animals were fasted overnight before the trials, but had free access to water. The use of experimental animals was evaluated and approved by the Animal Care Committee.
The otoscopy were performed in order to evaluate the external ear canal and the tympanic membrane. Experimental animal was placed in to the wooden-glass chamber (60 × 30 × 35 cm; L × W × H) with the openings for the wires of subcutaneous needle electrodes for recording of brainstem auditory evoked potentials (BAER), electroencephalography (EEG) and electrocardiography (ECG). Brainstem auditory evoked potentials were recorded on every side twice before euthanasia. Simultaneously EEG and ECG recording of 5 minute duration were performed before euthanasia with exhaust CO (concentration 4% in killing box) in order to record the alive EEG pattern [9] (Korhonen et al., 2011). The animal’s heart rate, breathing, palperal, corneal and withdrawal reflexes were examined after discontinuation of the BAER, EEG and ECG recordings and after removal of the animal from the chamber.
3. Results
According to the initial plan the first animal was sedated with an i.m. injection of medetomidine (Dorbene 0.20 ml, 200 micrograms). Additional two doses in the intervals of 5 minutes were injected i.m. as sufficient level of sedation was not reached. Animal never got sufficiently sedated and therefore experimental measurements were not possible. Based on this knowledge the next two animals were injected with 0.8 ml of medetomidine (Dorbene 0.80 ml, 800 micrograms) at once. Animals got sufficiently sedated to make manipulation, otoscopy and placing of the subdermal electrodes possible, but animals woke up after 86 and 53 seconds of measurement respectively and therefore the experiment needed to be interrupted. The next animal was injected with 1 ml of medetomidine (Dorbene 1 ml, 1 mg) at once. Also this fourth animal never got sufficiently sedated and therefore experimental measurements were not possible. The fifth and sixth animals were sedated with combination of 0.8 mg medetomidine and butorphanol (Butador) (2 mg in one mink and 4 mg in another) these animals got sedated to some extent so that the electroencephalography recording was possible, but BAER recording was not performed as animals did not tolerate sound and placing of electrodes.
As the initial planned sedation protocol appeared to be insufficient in the first six pilot study animals the anaesthesia protocol was amended and in all the further animals (N = 9) the combination of the 0,4 mg medetomidine (Dorbene 0.4 ml, 400 micrograms) and 10 mg tiletamine with 10 mg zolazepam (Zoletil 0.2 ml) was used instead. The combination of Dorbene (0.4 ml) and Zoletil (0.2 ml) were mixed within one syringe and injected intramuscularly in all animals. All the rest of mink got the same combination of anesthetics and all animals reached sufficient level of sedation in order to allow manipulation, otoscopy, placing of the electrodes and measurements throughout the experiment. The further description will concern the 9 mink where complete measurements were possible and only these animals were included in the further evaluation.
The heart rate ranged between 90 - 294 beats per minute (mean 194) and breathing frequency was 16 - 72 breaths per minute (mean 40). Palpebral, corneal and withdrawal reflexes were absent in all animals before placing of them in to the wooden-glass euthanasia chamber.
Figure 1 shows an example of normal EEG, ECG and respiratory pattern in sedated mink. After 39 sec from CO gas input first pathological EEG changes were seen (Figure 2). Respiration and ECG are still normal here. Changes in ECG were seen 120 sec from the gas input (Figure 3). 100 and 190 sec from start EEC and respiration, respectively, were gone (Figure 4). ECG showed still pulsless activity and was finally gone since 168 sec from the start og gas input. In all proper sedated mink (N = 9), first signs of EEG changes were found 42 ± 10 sec from gas input. EEG was totally lost 86 ± 35 sec. Correspondingly, first sign of changes in respiration and ECG were seen 42 ± 17 and 105 ± 37 sec from start of CO exposure. Respiration and ECG were totally gone in 217 ± 53 and 292 ± 130 sec, respectively.
4. Discussion
The mink is a very aggressive animal that require chemical immobilization or anaesthesia for most clinical pro-

Figure 1. Example of normal electroencephalography (EEG), electrocardiography (ECG) and breathing pattern in sedated farm-raised male mink. Background activity is superimposed with sleep spindles (see arrows C3-C4). Bar: sensitivity 50 uV/cm, 1 sec 3 cm.
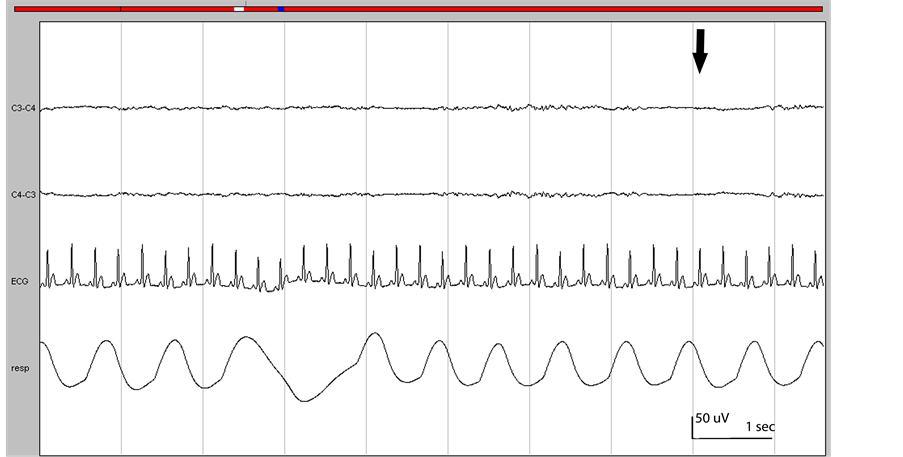
Figure 2. First pathological EEG changes. Burst suppression (arrow). Respiration and ECG are normal. Bar: sensitivity 50 uV/cm, 1 sec 3 cm.

Figure 3. ECG changes. ECG amplitude and frequency is decreasing. EEG is not present (isoelectric line) and respiration has severely decreased frequency. Bar: sensitivity 50 uV/cm, 1 sec 3 cm.

Figure 4. EEG and respiration is not present (isoelectric lines) and ECG shows pulsless activity. Bar: sensitivity 50 uV/cm, 1 sec 3 cm.
cedures. Most of the drugs that have been previously tested have disadvantages which render them less suitable in this species [5] [9] . Therefore, further development of sedation procedures is essential.
The results of the current experiment indicate that the electrophysiological recordings are possible to perform in the mink in a similar way as in other animal species [7] . If animal is adequately sedated recording is technically straightforward. The beginning of our experiment showed clearly that the sedation with pure alpha 2 agonist medetomidine is not sufficient for mink species as two animals never got sedated at all and 2 animals got sedated too superficially and started moving during the experiment. In addition, the neuroleptanalgesia in combination of medetomidine and butorphanol is not sufficient to manipulate the animals safely for the examiner and the animal. Therefore the sedation protocol of the current experiment needed to be amended and combination of sedative (medetomidine), tranquilizer (zolazepam) and general anaesthetic (tiletamine) was needed for adequate anaesthesia. All nine animals were sufficiently anesthetized [1] as documented by absent corneal, palpebral and withdrawal reflexes in all animals and the vital parameters such as breathing and heart rate stayed in reference range.
Euthanasia should result in rapid loss of consciousness followed by cardiac or respiratory arrest and the ultimate loss of brain function [7] . Essential here is that euthanasia occurs with minimal pain and distress. Pain is that sensation that results from nerve impulses reaching the cerebral cortex via ascending neural pathways [2] Pain can be experienced only when the cerebral cortex and subcortical structures are functional [2] [10] . Therefore, the functional state of brain is essential for animal wellbeing during euthanasia.
According to the present results proper sedation with combination of medetomidine, zolazepam and tiletamine provided normal EEG, ECG and respiration pattern. Example of this was given in Figure 1. The mink were finally euthanized during sedation by using exhaust CO 4%. Follow-up of EEC, ECG and respiration pattern during pattern was successful (Figures 2-4), resulting decline of these parameters with logical manner [7] [9] . Our conclusion here is that the present sedation combination can be used for proper measurement of electrophysiology in mink.
5. Conclusion
The animals were successfully sedated with the combination of the 0.4 mg medetomidine (Dorbene 0.4 ml, 400 micrograms) and 10 mg tiletamine with 10 mg zolazepam (Zoletil 0.2 ml). The combination of Dorbene (0.4 ml) and Zoletil (0.2 ml) were mixed within one syringe and injected intramuscularly. The rest of mink got the same combination of anesthetics and all animals reached the sufficient level of sedation to measure properly electroencephalography (EEC), electrocardiography (ECG), respiratory rate and brainstem auditory evoked responses (BAER).
Acknowledgements
This study was financially supported by the Ministry of Agriculture and Forestry (Finland) and the European Fur Breeders’ Association (EFBA). Heli Lindeberg, Pekka Toikkanen, Pekka Eskeli and Juhani Sepponen are greatly acknowledged for their valuable assistance in carrying out these experiments.