The Anti-Plasticization Effects of Dimethyl Carbonate Plasticizer in Poly (Methyl Metacrylate) Electrolyte Containing Rubber ()
1. Introduction
Plasticizer is defined as a relatively low molecular weight substance of low volatility, which, when added to another material, changes the physical and chemical properties of the material in such a manner that the finished product is in a more useful form [1]. In polymer electrolytes, plasticizers or mixed plasticizers are added to soften rigid polymers and to lower the glass transition temperature, Tg, of a polymer or polymer blend by increasing the segmental motion of the polymer backbone, hence assisting the transport of ions along the polymer chain. Furthermore, the addition of plasticizers helps to increase the dissolution of salts and dissociation of ion pairs and hence increase the number of free ions.
To date, there are several plasticizers that had been used as a plasticizer for the above mentioned purposes, such as ethylene carbonate (EC) [2], propylene carbonate (PC) [3], dimethyl carbonates (DMC) [4], dimethyl formamide (DMF) [5], etc. Amongst them, the most widelyused plasticizers are EC and PC, due to their low molecular weight, low viscosity, high dielectric constant and high boiling point properties.
However, not all plasticizers are found to be suitable in any polymer electrolyte system resulting to poor ionic conductivity. According to Bernardo and Burell[1], antiplasticization effects of plasticizer may occur in certain amount of plasticizer. This phenomenon exists in our previous work [6] in which the addition of EC reduced the ionic conductivity of PMMA/ENR 50/LiCF3SO3 from 10−6 S/cm to 10−8 S/cm at room temperature. In this EC plasticized system, we found that it was incompatible with epoxidised natural rubber in which it caused the rubber to be coagulated, hence reduced the ionic conductivity of the unplasticized system. The high dielectric constant of EC (ε = 95) may increase the number of “free” mobile ENR 50 chains resulting to chain entanglement hence leading to the formation of coagulates. Since ENR 50 was able to enhance the ionic conductivity (10−5 S/cm) [7] and improve the mechanical strength and adhesion properties of PMMA film electrolyte, it is important to further improve the ionic conductivity of the PMMA film since it was proven to exhibit good interfacial properties towards the lithium electrodes [8,9]. Therefore, DMC plasticizer that has much lower dielectric constant (ε = 3) than EC was chosen in this study to avoid the formation of excessive ENR 50 coagulation that hinders the migration of ions in the blend system. Though DMC also exhibited lower ionic conductivity than the unplasticized blend system, the phenomenon that occurred in this DMC plasticized system was not the same as in the EC plasticized system. To the best of our knowledge, DMC plasticizer may exhibit a high ionic conductivity of 10−3 S/cm at room temperature in other PMMA-based electrolyte system [10] but not when ENR 50 was blended with it. Therefore, this work emphasized the factors that occurring in this kind of polymer blend system.
2. Experimental
2.1. Film Preparation
(ALDRICH) and dimethyl carbonate, DMC plasticizer (BDH) were used without further purification. ENR 50 was obtained from Guthrie Polymer Sdn. Bhd. Siliau, Negeri Sembilan, Malaysia. PMMA and ENR 50 stock solutions were prepared separately by dissolving the polymers in THF by continuous stirring with magnetic stirrer. Fixed volume of the two polymer solutions were then mixed in a beaker containing fixed amount of LiCF3SO3 salt. Various amounts of DMC plasticizer was added into the solution mixture. The mixtures were then stirred for about 24 hours. All the preparation steps were done in a glove box. The electrolyte solutions were then cast into glass petri dishes and left to dry by solvent evaporation at room temperature. The films obtained were further dried in an oven at 50˚C for another 48 hours. The solvent free films were then kept in a desiccator until further use.
2.2. Material Characterizations
The morphology of the films was investigated under LEO Field Emissions Scanning Electron Microscope. The FTIR spectra of the thin films was obtained from SHIMADZU FTIR 8300 Fourier Transform Infra Red Spectrophotometer in the frequency range of 4000 - 400 cm−1. The conductivity measurement was performed by Hioki 3532 - 50 LCR HiTester Impedance Spectroscopy over a frequency range of 100 Hz to 1 MHz from room to 359 K.
3. Results and Discussions
All DMC plasticized PMMA/ENR 50/LiCF3SO3 electrolyte films were transparent and stable at room temperature. Interestingly, though the phase separation can still be observed on the surface of the films, it was almost diminishing and insignificant.
3.1. FESEM Studies on the Morphology of Plasticized PMMA/ENR 50/LiCF3SO3 Films
From observation, as the volume of DMC plasticizer increased, the film became congested (Figure 1(b)) with dissolved lithium salt that spread in the entire volume of the blend hence reduced the gap between the PMMA and the ENR 50 phase. This may explain why at higher volume of DMC plasticizer, the two phases became almost invisible and a physically better appearance of the blend film was obtained. Large craters were formed in the SEM micrographs of the blend when DMC plasticizer was added (Figure 1). The formation of craters were also
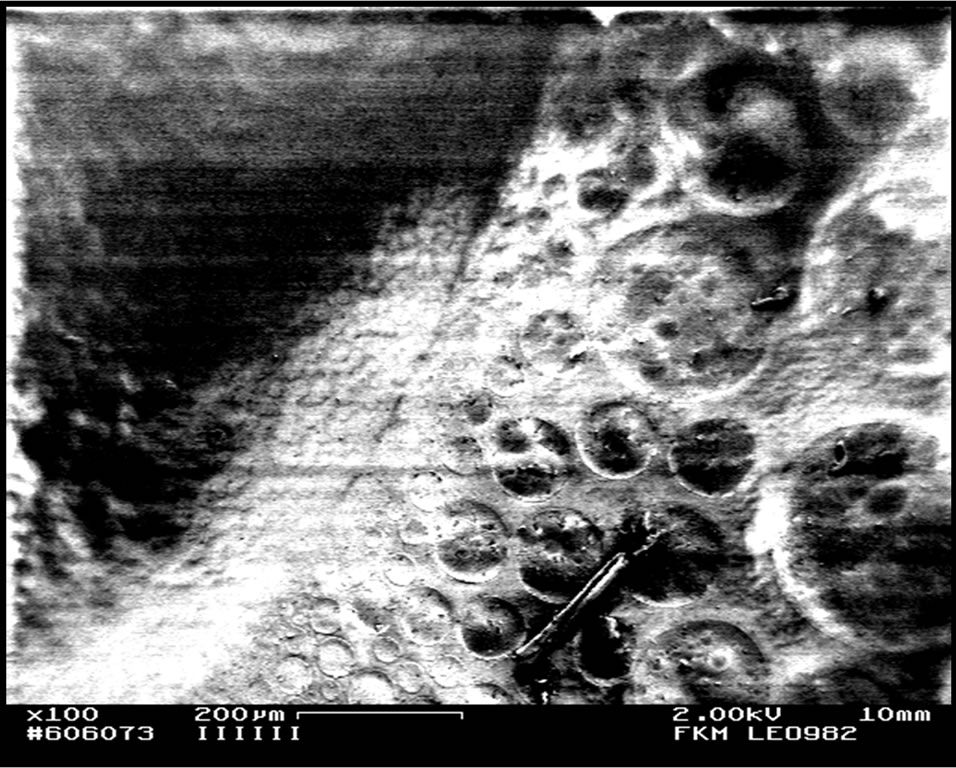 (a)
(a)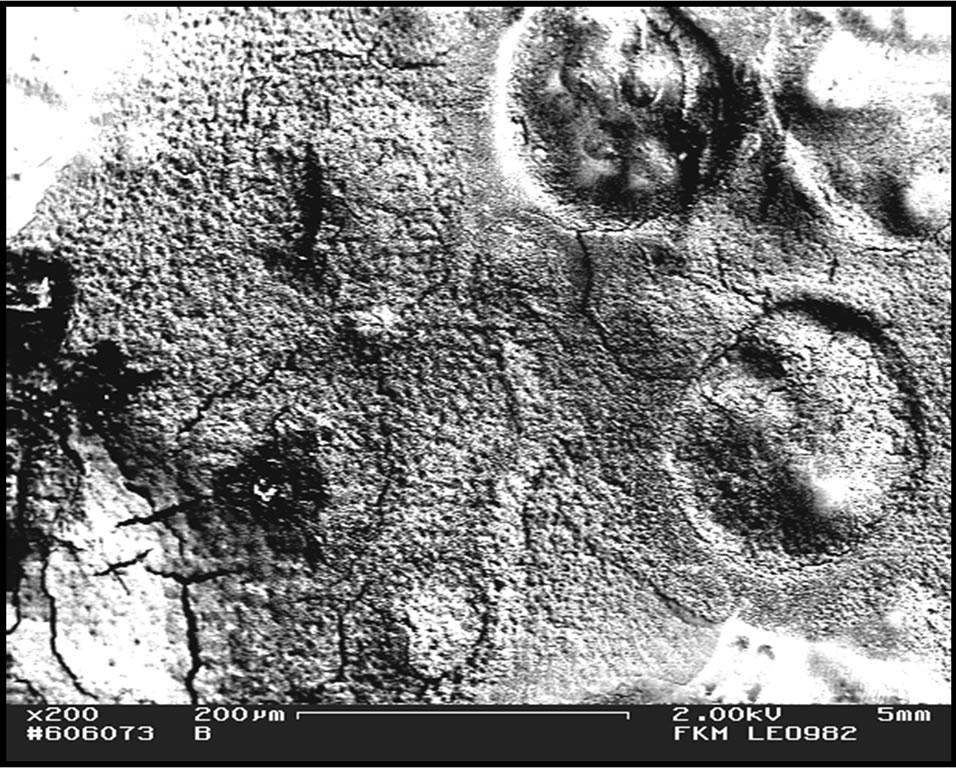 (b)
(b)
Figure 1. FESEM micrographs for PMMA/ENR 50/LiCF3SO3 films when plasticized with (a) 1 ml and (b) 3 ml of DMC plasticizer.
observed in the un-doped blend [6] when ENR 50 suppressed the globular structures of PMMA [6] as it penetrated the PMMA phase. The larger craters that were formed in these plasticized blends support the increment in the polymers chains flexibility that allows them to merge into each other’s phase. Therefore, more globular structures of PMMA were suppressed by ENR 50.
3.2. FTIR Analysis on Plasticized PMMA/ENR 50/LiCF3SO3 Electrolytes
It was found that the intensity of the –OH band at ~3500 (Figure 2) that relates to the occurrence of H-bonding have been reduced in the presence of plasticizer indicating a reduction in the number of interchain crosslinking between the two polymers. This confirmed that the addition of plasticizer increased the polymers chains flexibility.
It was found that the intensity of Vs (CF3) and Vs (SO3) at ~1350 cm−1 and ~1033 cm−1 peaks were reduced when DMC plasticizer was added into the system (Figure 3) indicating a marked reduction in the number of free ions due to the formation of ion pair or ion aggregates as a result of ion congestions. Therefore, there was no significant shifting and change in the intensity of the carbonyl peak of PMMA at ~1720 cm−1 (Figure 3) as the amount of DMC plasticizer was increased hence suggesting that no further complexation that occurred between the polymer and the salt.
The reduction in the intensity of the epoxy band of ENR 50 at ~1250 cm−1 and ~838 cm−1 (Figure 3) and the disappearance of the carbonate band of the plasticizer at ~1759 cm−1 (Figure 3) may suggest the occurrence of polymer-plasticizer interaction via polar linkages [6] due to its affinity towards ENR 50. However, this polar
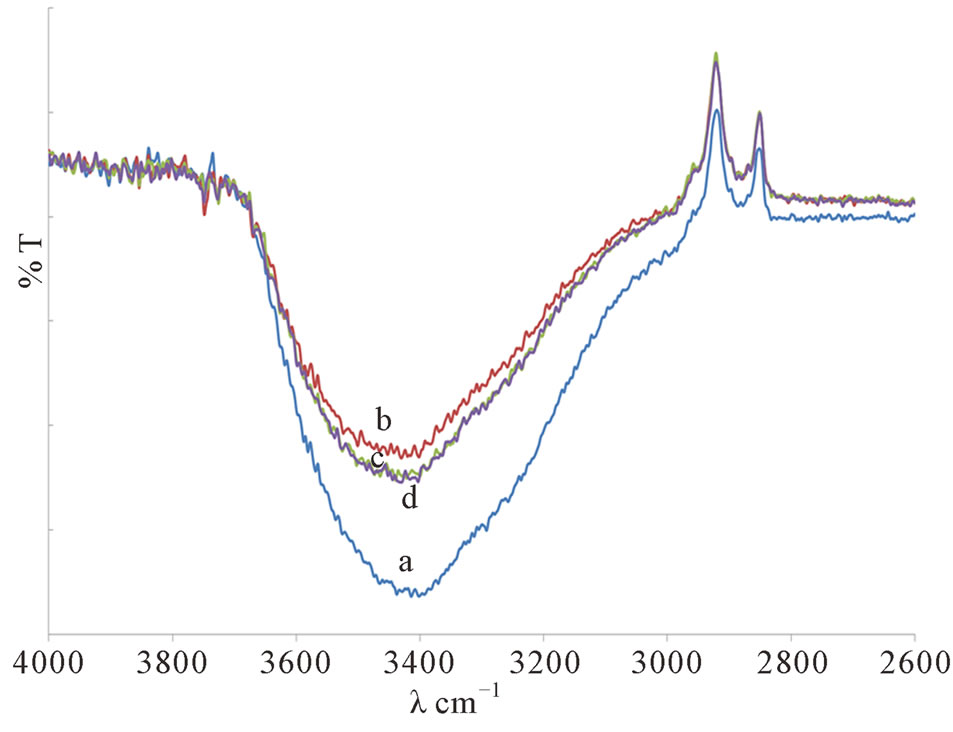
Figure 2. The FTIR spectra of the –OH band for PMMA/ ENR 50/LiCF3SO3 Films when plasticized with (a) 0 ml, (b) 1 ml, (c) 2 ml and (d) 3 ml of DMC.
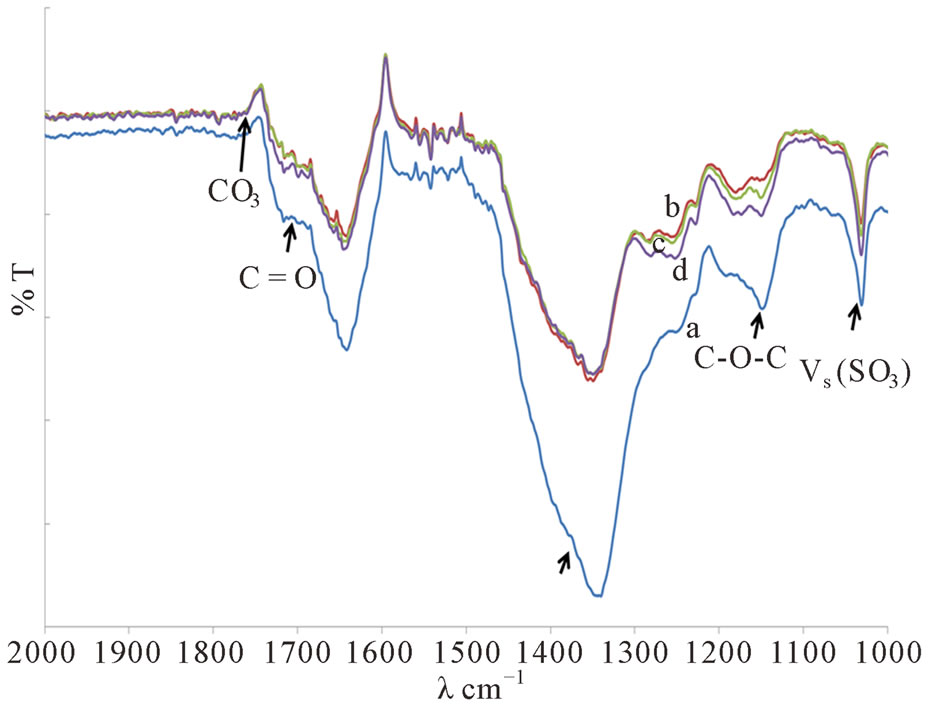
Figure 3. The FTIR spectra of various bands in PMMA/ ENR 50/LiCF3SO3 Films when plasticized with (a) 0 ml, (b) 1 ml, (c) 2 ml and (d) 3 ml of DMC.
linkages are weaker than the inter-or intra molecular hydrogen bonding.
3.3. Ionic Conductivity Studies on Plasticized PMMA/ENR 50/LiCF3SO3 Electrolytes
Table 1 summarized the ionic conductivity of these plasticized PMMA/ENR 50/LiCF3SO3 electrolyte systems. The data obtained in Table 1 supports the results obtained in the FTIR analyses where the conductivity obtained for the plasticized systems is lower than the unplasticized system due to the lower number of the charge carrier due to the formation of ion congestion that was observed in the SEM micrograph of the plasticized system at higher amount of plasticizer. The presence of craters may also trap the lithium ion in its vicinity hence hindering the migration of ion. These may also explain why the activation energies, Ea obtained from these plasticized systems are slightly larger than the un-plasticized system (Table 1).
Though various factors contribute to the lower ionic conductivity of this DMC plasticized PMMA/ENR 50 LiCF3SO3 system, it is still slightly higher than the EC plasticized system (3.84 × 10−7 S/cm) [6] due to no large coagulate structures were formed in the system.
Since the regression, r2 value for all the plots of ln (σ) versus 1000/T (Figure 4) for these plasticized system lie in the range of 0.96 to 0.999, therefore, it can be considered that the points were almost in a straight line. This implies that the conductivity behaviour of this system as a function of temperature can be fitted by the Arrhenius law in which the ion transport was similar to that in ionic crystals.
4. Conclusion
The addition of DMC plasticizer in PMMA/ENR 50/
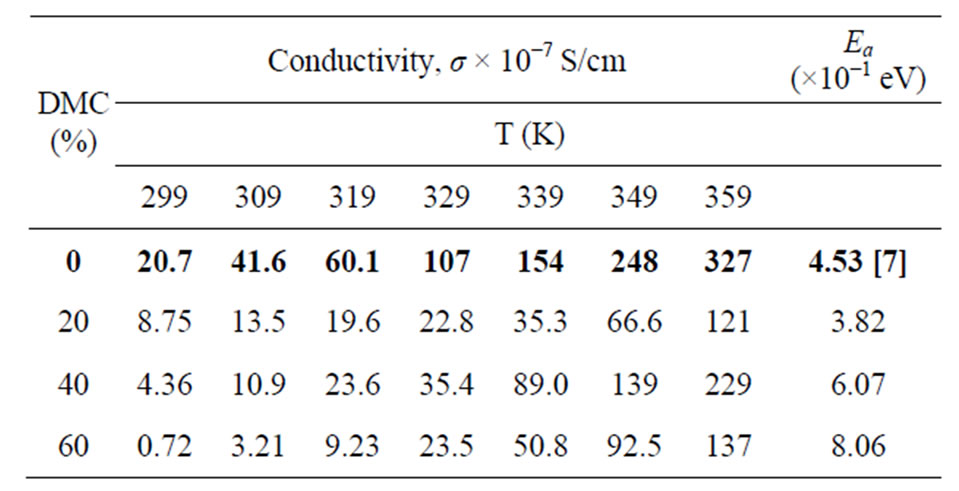
Table 1. The average conductivity of PMMA/ENR 50/ LiCF3SO3/DMC electrolytes at various amounts of DMC and temperatures.
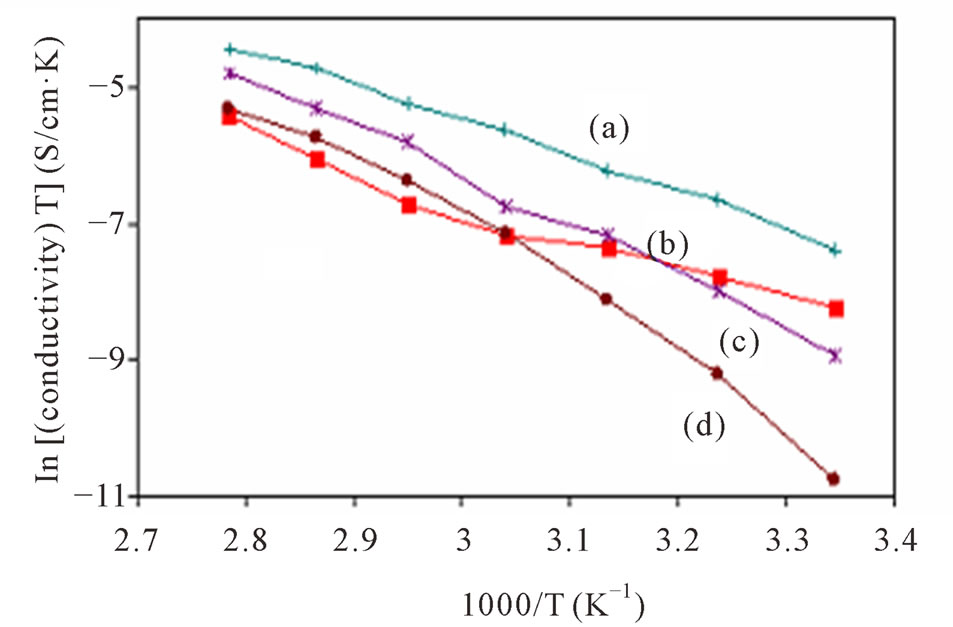
Figure 4. Arrhenius plot for PMMA/ENR 50/LiCF3SO3 electrolyte when plasticized with (a) 0 ml [11], (b) 1 ml, (c) 2 ml and (d) 3 ml of DMC.
LiCF3SO3 was found to reduce the ionic conductivity of the unplasticized electrolyte because it contained lower number of charge carrier due to the formation of ion pairs and ion aggregates or trapping of charge in the vicinity of the craters. Therefore, it can be concluded that the value of dielectric constant of a plasticizer is important when dealing with polymer electrolyte system containing ENR 50.
5. Acknowledgements
Financial support from IRPA, FRGS grants and technical support from Universiti Teknologi MARA are highly acknowledged. Thanks to Guthrie Polymer Sdn. Bhd. Siliau, Negeri Sembilan, Malaysia for providing free samples of ENR 50.