Morphine has latent deleterious effects on the ventilatory responses to a hypoxic challenge* ()
1. INTRODUCTION
Opioids depress minute ventilation (Vm) and disturb arterial blood gas (ABG) chemistry and Alveolar-arterial (A-a) gradients in humans via central and peripheral effects [1-4]. The ability of morphine to depress the ventilatory responses of humans to hypoxic [5-8] and hypercapnic [8] challenges, strongly suggests that opioids depress carotid body mechanisms responsive to these challenges [9,10] and central mechanisms responsive to hypercapnia [7,8]. Evidence that the metabolite, morphine- 6-glucurodide (M6G), blunts the ventilatory response to hypercapnic challenge in humans without affecting resting ventilation [5] poses the unaddressed question as to whether analgesic doses of morphine have latent deleterious effects on ventilatory systems responding to hypoxic/hypercapnic challenges. This is an important question since patients are deemed to have recovered from the acute ventilatory depressant effects of morphine when respiratory rate and ABG chemistry are normal. However, if morphine was still able to depress ventilatory responses to hypoxic challenge, patients may be prone to respiratory collapse because chemoafferent systems fail to trigger appropriate ventilatory responses.
Animal studies have provided extensive insight into the mechanisms by which opioids such as morphine depress ventilation and ABG chemistry [11-15]. The mechanisms include 1) centrally-mediated depression of ventilatory drive [16], 2) skeletal muscle rigidity in the chestwall [17], 3) increases in airways resistance [18,19], and 4) an increase pulmonary vascular resistance, which suggests decreased perfusion of alveoli [20]. Opioids also blunt the hypoxic ventilatory response in rats [21] and inhibit carotid body chemoafferent responses to hypoxia and hypercapnia [22-24]. As with humans, it is not known whether morphine has latent deleterious effects on ventilatory control systems in the rat. As such, our aim was to determine whether morphine depressed the ventilatory responses of conscious rats elicited by hypoxic challenges given when the effects of morphine on ABG chemistry, A-a gradient and Vm had completely subsided.
2. METHODS
2.1. Rats and Surgeries
All studies were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996. The protocols were approved by the Animal Care and Use Committee of the University of Virginia. Adult male Sprague-Dawley rats (Harlan, Madison, WI, USA) were implanted with jugular vein and femoral arterial catheters (ABG studies) or jugular vein catheters only (ventilation studies) under 2% isoflurane anesthesia. The rats were allowed 4 days to recover from surgery before use. All catheters were flushed with saline at least 4 h before commencement of experiments. All studies were performed in a quiet room with relative humidity of 52% ± 4% and room temperature of 21.3˚C ± 0.2˚C.
2.2. Analgesia Assay
Analgesia was assayed using Hargreaves thermal sensitivity method [25,26]. The rats were placed into plexiglass animal enclosures surrounding a 30˚C heated glass platform and were allowed to acclimate for at least 30 min. A radiant heat source was activated with a timer and focused onto the animal hindpaw. Paw-withdraw latency was determined by a detector that halted both lamp and timer upon paw withdrawal. Lamp intensity was adjusted to obtain average baseline withdrawal latencies of 20 sec, to allow for detection of possible hyperalgesia. A cutoff of 40 sec was used to prevent tissue damage.
2.3. Blood Gas Measurements and Determination of Arterial-Alveolar Gradient
pH, pCO2, pO2 and sO2 of arterial blood samples (100
µL) were measured by a Radiometer Blood-gas Machine (ABL800 FLEX). The A-a gradient measures the difference between alveolar arterial blood pO2 and is an index of ventilation-perfusion in the lung [27,28]. An increased gradient reflects an abnormally low pO2 in lung blood versus alveoli. A decrease in PaO2, without a change in A-a gradient is caused by hypoventilation whereas a decrease in PaO2 with an increase in A-a gradient indicates ventilation-perfusion mismatch or shunting [27,28]. The formulas used to derive A-a gradient are shown in the legend to Table 1.
2.4. Ventilatory Parameters
Ventilatory parameters were continuously recorded in conscious unrestrained rats using a whole-body plethysmography system (PLY 3223; BUXCO Incorporated, Wilmington, NC, USA) [29,30]. The parameters were 1) frequency of breathing (fr), 2) tidal volume (Vt), 3) minute volume (Vm), 4) inspiratory time (Ti), 5) expiratory time (Te), 6) end inspiratory pause (EIP), time between end of inspiration and start of expiration, 7) peak inspiratory flow (PIF), and 8) peak expiratory flow (PEF). Vt/Ti, an index of Respiratory Drive [31], was calculated from the recorded Vt and Ti, data. The provided software constantly corrected digitized values for changes in chamber temperature and humidity and a rejection algorithm was included in the breath-by-breath analysis to exclude motion-induced artifacts. Due to the closeness of body weights in the experimental groups, ventilatory data are presented without corrections.

Table 1. Percent changes in arterial blood gas chemistry and Alveolar-arterial gradient elicited by injections of vehicle or morphine.
2.5. Body Temperature Measurements
Body temperatures (Bt) of male Sprague Dawley rats were measured via telemetry [32]. Rats were anesthetized (2% isoflurane) and a telemetry probe (ETA-F20, Data Sciences International, MN) was implanted into the peritoneal cavity. A jugular vein catheter was also implanted. After 4 days, the rats were placed in plethysmography chambers, under each of which was placed a telemetry receiver connected via a data exchange matrix to a computer. The Bt signals were sampled once a minute using internal hardware and Dataquest ART Gold3.1 software. Digitized signals were transferred to MATLAB (MathWorks, Natick, MA) for analysis.
2.6. Protocols for Analgesia Studies
Paw-withdraw latencies were determined in a group of 4 rats (290 ± 4 grams) before and 20, 40, 60 and 90 min after a bolus injection of morphine (5 mg/kg, i.v.). All studies used an injectable form of (+)-morphine sulfate (10 mg/ml) from Baxter Healthcare (Deerfield, IL, USA).
2.7. Protocols for Arterial Blood Gas and Alveolar-Arterial Gradient Studies
Arterial blood sample (100 µL) were taken before (2 - 3 samples and values averaged) and 3, 6, 9, 12, 15 and 30 min after bolus injections of vehicle (saline, n = 8 rats; 293 ± 3 grams) or morphine (5 mg/kg, i.v., n = 8 rats; 291 ± 3 grams).
2.8. Protocols for Ventilation Studies
The rats were placed in the plethysmography chambers and allowed 45 - 60 min to acclimatize. Data was continuously recorded (i.e., breath by breath) throughout the acclimatization period (the last 15 min was used for analysis and graphing) and subsequent experimentation. The data were collected into 1 min bins for analysis and graphing. Two groups of rats were used in these studies. One group of rats (n = 12, 281 ± 1 grams) received an injection of vehicle (saline, i.v.) whereas another group (n = 12, 284 ± 3 grams) received an injection of morphine (10 mg/kg, i.v.). After 15 min, the rats were exposed to 3 consecutive 15 min episodes of hypoxia (10% O2, 90% N2), each of which was separated by a 15 min exposure to room air.
2.9. Protocols for Body Temperature Studies
Rats with jugular vein catheters and telemetry devices were placed in plethysmography chambers and allowed 45 - 60 min to acclimatize. One group (n = 6, 303 ± 2 g) received an i.v. injection of vehicle (saline) whereas another group (n = 6, 304 ± 3 g) received morphine (5 mg/kg, i.v.). After 15 min, the rats underwent the hypoxia-normoxia challenges as described above.
2.10. Statistics
Data recorded during the ventilatory studies (1 minbins) and derived parameters, namely, TV/Ti and cumulative response (cumulative arithmetic changes from pre-values), were taken for analyses. All data are presented as mean ± SEM and were analyzed by one-way or two-way ANOVA followed by Student’s modified t test with Bonferroni corrections for multiple comparisons between means [33]. P < 0.05 denoted significance.
3. RESULTS
3.1. Analgesia
Paw-withdraw latencies in rats prior to injection of morphine (5 mg/kg, i.v.) were 18.9 ± 0.5 sec. The paw withdrawal latencies at 20, 40, 60 and 90 min after morphine injection were 40 ± 0, 40 ± 0, 39.4 ± 0.6 and 38.9 ± 1.1 sec, respectively (P < 0.05, for all comparisons to pre-injection values). Remembering that the cutoff-latency was 40 seconds, it was evident that of morphine elicited a sustained analgesia in these rats.
3.2. Arterial Blood Gas and Alveolar-Arterial Gradient Studies
The effects of vehicle or morphine (5 mg/kg, i.v.) on ABG chemistry and A-a gradient are summarized in Figure 1 and Table 1. Vehicle did not affect these parameters whereas morphine elicited prompt decreases in arterial blood pH, pO2 and sO2 and increases in pCO2 and A-a gradient. All responses were resolved by 15 min and no changes were observed at 30 min.
3.3. Ventilatory Responses Elicited by Morphine
Morphine (5 mg/kg, i.v.) elicited transient increases in fr which were associated with decreases in Vt and Vm of 9 - 10 min in duration (Figure 2, Table 2). Despite the transient nature of the morphine-induced increase in fr, morphine elicited sustained increases in Ti and EIP but a sustained decrease in Te (Figure 3, Table 2). In addition, morphine elicited sustained decreases in Vt/Ti and PIF but relatively transient changes in PEF (Figure 4, Table 2). Vehicle elicited minor responses (Figures 2-4, Table 2). Taken together, vehicle elicited minor cumulative responses whereas morphine elicited significant cumulative responses in all parameters except fr and PEF (Table 3).
3.4. Ventilatory Responses during Hypoxic Challenges and upon Return to Room-Air
fr, Vt and Vm: Episode 1 of hypoxia (H1) elicited rapid
and sustained increases in fr, Vt and Vm in vehicletreated (VEH) rats (Figure 2, Table 4). The increases in fr and Vm developed more slowly during H2 and H3. In morphine-treated (MOR) rats, H1-H3 transiently increased fr (Figure 2, Table 4). The increases in Vt during H1 were similar to those in VEH rats whereas the increases during H2 and H3 were greater. The increases in Vm during H1 in MOR rats were smaller than in VEH rats (due to lesser increases in fr) whereas the increases in Vm during H2 and H3 were similar in both groups. In VEH rats, fr returned to pre-levels during episode 1 of normoxia (N1) but fell below pre-values for several min during N2 and N3. Vt and Vm dipped below pre-levels during N1 - N3 but normalized within 5 - 10 min. In MOR rats, fr changed minimally during N1 whereas it rose and remained elevated during N2 and N3. Vt tended to return to pre-levels during N1 - N3 in MOR rats. Vm returned to pre-levels during N1 - N3 in MOR rats but remained above pre-levels during N2 and N3. fr and Vm values in MOR rats were higher than in VEH rats at the end of N3 (Figure 2).
Ti, Te and EIP: H1 - H3 elicited sustained decreases in Ti, Te and EIP in VEH rats (Figure 3, Table 4). H1 - H3 elicited sustained decreases in Ti in MOR rats (Figure 3, Table 4) but due to higher baseline values, nadir values did reach those in VEH rats. In contrast, Te (depressed by morphine) tended to recover to pre-levels during H-H3. Moreover, EIP (elevated by morphine) rapidly returned to pre-levels during H1 - H3. In VEH rats, Ti and EIP returned to baseline during N1 - N3 (Figure 3, Table 4). Te rapidly returned to and exceeded pre-values during N1 - N3 but returned to pre-levels before each episode of hypoxia (Figure 3, Table 4). In MOR rats, Ti rose rapidly upon return to room-air during N1, less so during N2 and minimally during N3. Te fell back to (morphine-induced) nadir levels during N1 - N3. EIP returned to the elevated levels elicited by morphine during N1 - N3. Ti and EIP values in MOR rats were
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Changes in frequency of breathing (a), tidal volume (b) and Minute Volume (c) elicited by injections of vehicle or morphine (5 mg/kg, i.v.) and subsequent exposure to three 15 min episodes of hypoxia (10% O2, 90% N2) each of which was followed by a 15 min period of normoxia (room-air). The first episode of hypoxia began 15 min after injection of vehicle or morphine. There were 6 rats in each group. Data are mean ± SEM.

Table 2. Ventilatory responses elicited by vehicle or morphine.
 (a)
(a) (b)
(b) (c)
(c)
Figure 3. Changes in inspiratory time (a), expiratory time (b) and end inspiratory pause (c) elicited by injections of vehicle or morphine (5 mg/kg, i.v.) and subsequent exposure to three 15 min episodes of hypoxia (10% O2, 90% N2) each of which was followed by a 15 min period of normoxia (room-air). The first episode of hypoxia began 15 min after injection of vehicle or morphine. There were 6 rats in each group. Data are mean ± SEM.

Table 3. Cumulative responses elicited by vehicle or morphine.
similar to VEH rats at the end of N3 whereas Te was decreased (Figure 3).
Vt/Ti, PIF and PEF: H1 - H3 elicited sustained increases in Vt/Ti in VEH and MOR rats (Figure 4, Table 4). Vt/Ti values in MOR rats did not reach those of VEH rats during H1 but approached them in H2 and equaled them in H3. In VEH rats, Vt/Ti rapidly returned to baseline levels during N1 - N3. In MOR rats, Vt/Ti returned to the lower morphine-induced baseline during N1. The decreases in Vt/Ti during N2 and N3 were similar in both groups. H1 - H3 elicited sustained increases in PIF in VEH and in MOR rats. The responses during H1 and H2 in MOR rats did not reach the maxima in VEH rats whereas the responses during H3 were equal to VEH rats. In VEH rats, PIF returned to pre-levels during N1 whereas it fell below pre-levels during N2 and N3. In MOR rats, PIF returned to post-morphine levels during N1 but returned to pre-morphine levels during N2 and N3. H1 - H3 elicited sustained increases in PEF in VEH and MOR rats (Figure 4, Table 4). The responses during H1 in MOR rats were smaller than in VEH rats. The responses during H2 and H3 were similar in both groups
 (a)
(a) (b)
(b)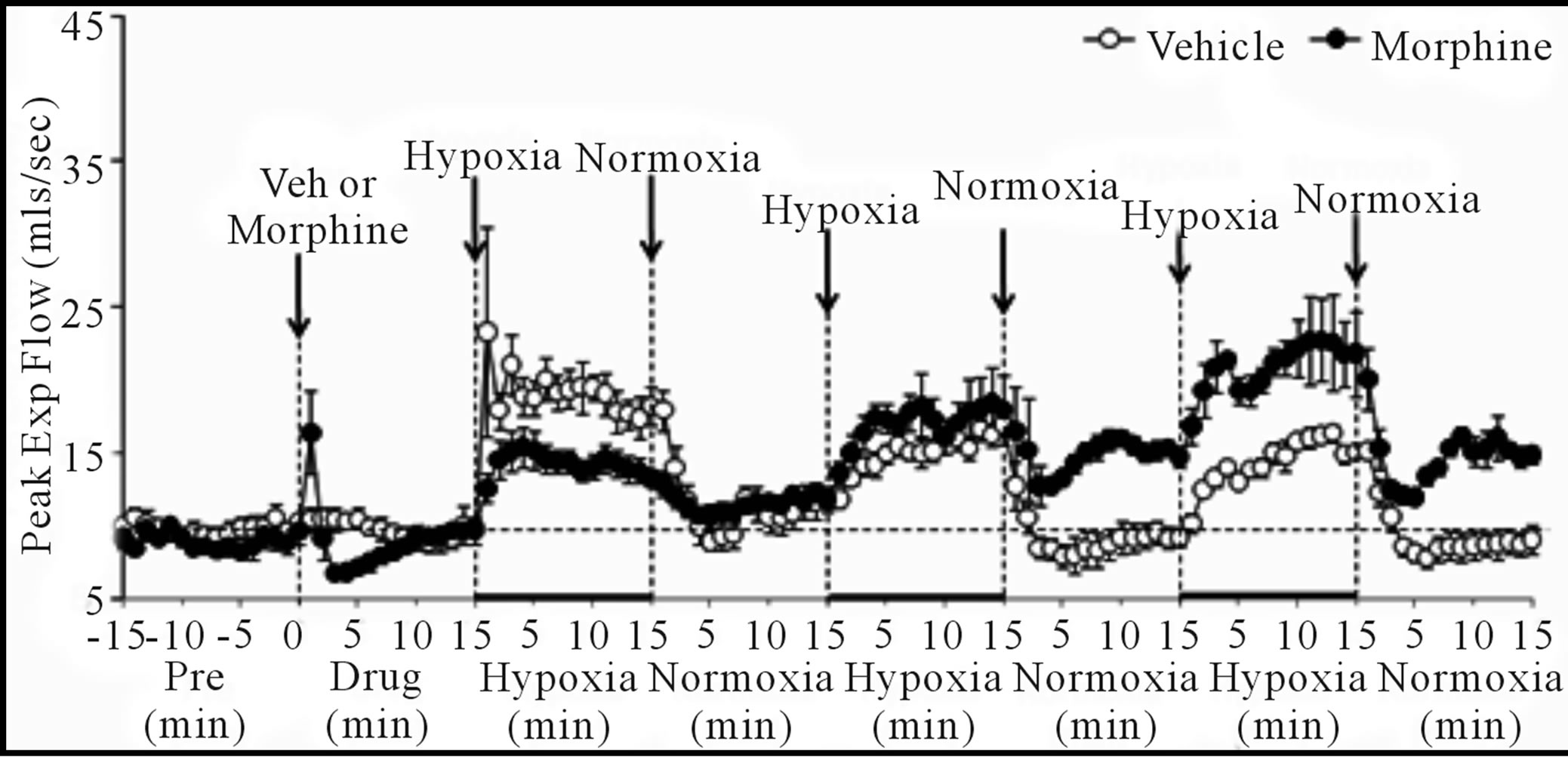 (c)
(c)
Figure 4. Changes in tidal volume/inspiratory time (a), peak inspiratory flow (b), and peak expiratory flow (c) elicited by injections of vehicle or morphine (5 mg/kg, i.v.) and subsequent exposure to three 15 min episodes of hypoxia (10% O2, 90% N2) each of which was followed by a 15 min period of normoxia (room-air). The first episode of hypoxia began 15 min after injection of vehicle or morphine. There were 6 rats in each group. Data are mean ± SEM.
although higher values were reached in MOR rats during H3 because of higher pre-hypoxia values. In VEH rats, PEF returned to pre-levels during N1 - N3. In contrast, PEF in MOR rats remained elevated during N2 and N3. Vt/Ti values were similar in VEH and MOR rats at the end of N3, whereas PIF was lower in VEH rats because of the sustained post-hypoxic depression (Figure 4). PEF was higher in MOR rats at the end of N3 because of the sustained effects of morphine (Figure 4).
Cumulative responses: In VEH rats. H1 - H3 elicited similar cumulative responses in Vt, Ti, Te, and EIP whereas those in fr, Vm, Vt/Ti, PIF, and PEF gradually diminished with each episode (Table 5). H1 - H3 elicited markedly different cumulative responses in MOR rats than in VEH rats, and the magnitude of these differences changed with each hypoxic episode (Table 5). N1 - N3 elicited substantial cumulative responses in fr, Vm, Ti, EIP, PIF, and PEF but minimal responses in Vt, Ti, and Vt/Ti. Moreover, the magnitude and direction of these cumulative responses changed with each episode of normoxia. For example, N1 was associated with positive cumulative response for fr and Vm, whereas N2 and N3 were associated with negative responses. N1 - N3 also elicited substantial cumulative responses in MOR rats that changed markedly with each successive challenge (Table 5). Although the cumulative responses during N1 for fr, Vt, and Vm were similar in VEH and MOR rats, the cumulative responses in MOR rats greatly exceeded those in VEH rats during N2 and N3.
3.5. Body Temperature (Bt)
H1 - H3 elicited minor but sustained decreases in Bt in VEH rats (e.g., H1 at +15 min, −0.39˚C ± 0.04˚C, P < 0.05) (Figure 5). Bt returned to pre-hypoxia levels upon return to room-air (N1 - N3). Morphine elicited a minor increase in Bt, which was still evident at the time of exposure to H1 (+0.44˚C ± 0.08˚C, P < 0.05). H1 - H3 elicited minor decreases in Bt in MOR rats, although they never fell below pre-values. These decreases were still present at the end of each hypoxic challenge (e.g., H1 at +15 min, −0.55˚C ± 0.11˚C, P < 0.05). Bt returned to pre-hypoxia levels upon return to room air, such that the morphine-induced hyperthermia was sustained throughout the study.
4. DISCUSSION
Despite resolution of the morphine-induced changes in ABG chemistry, A-a gradient and Vm, the first episode of hypoxia elicited smaller ventilatory responses than in VEH rats. Moreover, the pattern of ventilatory responses during subsequent episodes of hypoxia changed in MOR rats. As such, morphine and/or metabolites have latent deleterious effects on ventilatory responses elicited by

Table 4. Values (expressed as % change from Pre values) recorded at the 15 min time-point during each episode of hypoxia or normoxia.
hypoxic challenge. These latent effects may involve direct effects on carotid body and/or brain mechanisms processing the hypoxic responses rather than changes in metabolism.
4.1. Analgesia and Body Temperature
The ability of 5 mg/kg morphine to elicit a long-lasting analgesia validated our choice of this dose for the ventilatory studies. Our finding that a 5 mg/kg dose of morphine elicited a minor sustained hyperthermia of » 0.4˚C is consistent with findings from other laboratories [34,35]. Exposure of VEH rats to 15 min episodes of hypoxia (10% O2, 90% N2) elicited minor decreases in Bt of 0.4˚C, consistent with evidence that exposure of rats to 10% - 12% O2 for 15 min elicits no effects [36] or a decrease of »0.5˚C [37,38]. Since each episode of hypoxia elicited very similar arithmetic falls in Bt in VEH and MOR rats, it appears that despite elevating Bt, morphine does not impair the metabolic processes that cause hypoxia-induced hypothermia. Taken together, the morphine-induced changes in BT are likely to have had minimal effects on the ventilatory responses to hypoxia.
4.2. Morphine-Induced Changes in ABG Chemistry, A-Arterial Gradient and Ventilation
The effects of morphine on ABG chemistry (i.e., decreases in pH, pO2 and sO2, increases in pCO2) are consistent with hypoventilation (i.e., decreases in Vt) whereas the increases in A-a gradient (mismatch of ventilation-perfusion) may have arisen via increases in pulmonary airways resistance [18,19], pulmonary vasoconstriction reducing arterial blood flow to alveoli [20,39,40] and/or exacerbated hypoxic pulmonary vasoconstriction via morphine-induced reductions in arterial pO2. Although, morphine minimally affected fr, it lengthened Ti and EIP and so obviously affected inspiratory control mechanisms. Morphine also elicited 1) decreases in Vm via reductions in Vt that resolved by 15 min (i.e., the time of exposure to H1), 2) increases in Ti and EIP still evident at 15 min, 3) decreases in Vt/Ti and PIF still evident at 15 min, and 4) a transient decrease in PEF. The decreases in Vt and PIF suggest that morphine impaired ventilatory mechanical efficiency via decreases in neural drive to the intercostal muscles and diaphragm. Since the

Table 5. Cumulative responses during exposures to hypoxia and normoxia.
morphine-induced reductions in Vt/Ti had not resolved by 15 min, it appears that the negative influence on Respiratory Drive was present despite the resolution of ABG chemistry, A-a gradient and Vm. Morphine also elicited sustained decreases in Te (i.e., expiration was faster), which may have been due to diminished Vt requiring less time to exhale. However, the decreased Te may have involved direct (presumably central) effects of morphine on pathways driving expiration.
4.3. Ventilatory Responses to Hypoxic Challenges in Vehicle-Treated Rats
In VEH rats, the hypoxic challenges elicited prompt and sustained ventilatory responses including 1) increases in Vt/Ti and Vm (via increases in fr and Vt), 2) decreases in Ti and Te but minor decreases in EIP, and 3) increases in PIF and PEF. The rates of increase in fr, PIF, PEF, and Vt/Ti were slower during H2 and H3 than H1. This suggests that the mechanisms responsible for these rapid responses had “adapted” to hypoxic challenge whereas those involved in maintaining the responses did not. Whether this adaptation occurred in response elements sensitive to reductions in blood pO2 and in the carotid bodies [41-43], or within the brain is unknown. Ventilatory drive can diminish during exposures to severe hypoxia or to longer duration exposures to mild hypoxia. This “ventilatory roll-off” involves neurochemical processes in the NTS under mild hypoxia [44,45], and the direct depressive effects of severe hypoxia on brain neurons regulating ventilation [45,46]. However, in the present study, none of the key ventilatory parameters (e.g., fr, Vt, Inspiratory Drive) displayed of roll-off indicating that 15 min exposures to 10% O2 did not engender this phenomenon in our conscious Sprague-Dawley rats.
 (a)
(a) (b)
(b)
Figure 5. Changes in body temperature elicited by injections of vehicle (upper panel) or morphine (5 mg/kg, i.v., lower panel) and subsequent exposure to three 15 min episodes of hypoxia (10% O2, 90% N2) each of which was followed by 15 min periods of normoxia (room-air). The first episode of hypoxia began 15 min after injection of vehicle or morphine. There were 8 rats in each group. Data are mean ± SEM.
4.4. Ventilatory Responses to Hypoxic Challenges in Morphine-Treated Rats— Episode H1
H1 elicited markedly smaller increases in fr in MOR rats although resting fr was not diminished. As such, morphine elicited latent effects on systems including those within the carotid bodies that drive the hypoxic responses. Our data are consistent with evidence that the negative effects of opioids in humans may be latent since the morphine metabolite, M6G, does not affect resting ventilatory parameters whereas it substantially blunts the ventilatory response to hypercapnic challenge [5]. Since morphine did not markedly blunt the increase in Vt during H1, it is evident that it did not negatively affect neural drive to the chest muscles or diaphragm. This is supported by the finding that Ti in MOR rats (elevated immediately prior to exposure to hypoxia) decreased substantially during H1. Taken together, our data support the concept that morphine affected brainstem centers responsible for generating breathing such as the respiratory pattern central generator, including the pre-Botzinger complex [47,48], rather than affecting peripheral motor/ skeletal muscle components driving breathing. Nonetheless, it was evident that H1 elicited substantially smaller increases in PIF and PEF in MOR rats than in VEH rats thereby compromising the dynamics of active inspiratory/expiratory ventilation. It should be noted that resting PIF was still depressed in MOR rats (−24% ± 7%, P < 0.05) prior to H1 whereas resting PEF was not (+8% ± 6%, P > 0.05). In addition, Te was diminished prior to H1 in MOR rats (−23% ± 5%, P < 0.05) but actually rose appreciably during H1, suggesting that morphine inhibited the hypoxia-induced stimulation of active expiration.
4.5. Ventilatory Responses upon Reintroduction of Room-Air in Vehicle-Treated Rats
In humans, Vm falls below pre-hypoxia levels upon return to room-air via falls in fr and Vt ([49,50], 1994). In rats, fr falls below resting levels upon return to room-air via an increase in Te [51-53]. This post-hypoxia depression of fr (post-hypoxic fr decline. PHFD) [54], is an active neural process that depends on the ventrolateral pons [52,53,55,56]. In VEH rats, Ti, EIP, and PEF returned to pre-values upon return to room-air whereas fr, Vt, Vm and PIF declined below pre-levels. Consistent with previous findings [52,53], this PHFD was associated with an elongation in Te. The finding that the pattern of ventilatory responses was similar for each of the three normoxic episodes indicates that adaptations in carotid body or brainstem circuitry that may affect the normoxia responses did not occur during repeated exposure to hypoxia.
4.6. Temporal Changes in Ventilatory Responses to Hypoxia-Normoxia in Morphine-Treated Rats
The pattern of ventilatory responses in MOR rats changed during the study. Although fr during each episode of hypoxia was depressed in MOR rats, baseline fr gradually rose during N1 - N3 such that fr was higher than in VEH rats during N3. Since the PHFD observed in VEH rats was absent in MOR rats, morphine may have directly interfered with pontomedullary systems responsible for this phenomenon [52,53,55,56]. Although resting Vt returned to baseline levels during N1-N3 in MOR rats, the responses during H1 - H3 changed in that the increases in Vt following H1 were slightly greater than those of VEH rats whereas they were greater than VEH rats during H2 and H3. As a result of the temporal patterns of changes in fr and TV, resting Vm in MOR rats was substantially above that of VEH rats during N2 and N3. Moreover, although the peak increases in Vm in MOR rats were less than in VEH rats during H1, the increases in Vm in MOR rats were equal to those in VEH rats during H2 and reached higher values (perhaps due to elevated starting baselines) during H3. Although the PHFD in Vm appeared qualitatively similar in both groups, PHFD in VEH rats was due to a decline in fr and to a lesser degree a decrease in Vt whereas it was due solely to a decrease in Vt in MOR rats. The prolongation of Ti by morphine was essentially resolved when N3 was applied. Although each episode of hypoxia decreased Ti in the MOR rats, none of the decreases were as pronounced as those in VEH rats. Moreover, Ti promptly returned to elevated levels during N1 and N2 but not N3. Although morphine elicited reductions in Vt/Ti and PIF, which had not resolved prior to H1 or H2, these challenges still elicited robust increases in these parameters. In addition, although morphine elicited a sustained increase in EIP that was evident during N1 - N3, each episode of hypoxia elicited robust decreases in EIP. As such, it appears that whereas morphine reduced EIP, Vt/Ti and PIF, it did not depress the mechanisms by which the carotid bodies responded to hypoxia or central/efferent processing of this input. The findings that the PEF during N2 and N3 were elevated and that the post-hypoxia increases in Te in VEH rats (N1 - N3) were absent in MOR rats are suggestive of facilitatory effects of morphine and/or metabolites on expiratory control.
4.7. Mechanisms Responsible for the Delayed Effects of Morphine—Morphine Metabolites
The presence of (+)-morphine metabolites [57-60] may explain the delayed effects of the opioid on the ventilatory responses to hypoxia. In rats, maximal plasma levels of (+)-morphine occur within 10 min and the elimination half-life is approximately 45 - 50 min. As such, pharmacologically active plasma concentrations of morphine were likely present in our rats during H1 and N1 (15 to 45 min post-morphine injection), during H2 and N2 (46 - 75 min post-morphine) but perhaps less so during H3 and N3 (76 - 105 min post-morphine). It is feasible that morphine sequestered into relevant tissues may have effects when plasma levels are minimal. Obvious tissues would be 1) carotid bodies and neuromuscular elements of the chest wall [61], 2) brainstem nuclei receiving/processing carotid body chemoafferent input [62], and 3) brain structures devoid of blood-brain barriers [63]. It may also be possible that concentrations of morphine that are insufficient to affect central and/or peripheral drive may still be able to blunt the mechanisms responsible for sensing/expressing ventilatory responses to hypoxia. In humans, (+)-morphine is metabolized to M6G [57,60], which is relatively more selective for µ-opioid receptors (µ-ORs) than δ- or κ-ORs and elicits pronounced analgesia and respiratory depression [58,64]).
In rats, (+)-morphine is metabolized primarily to M3G [57,59,60]. Maximal plasma levels of M3G occur within 30 min of morphine administration and the elimination half-life is over 200 min. Unlike M6G, M3G has a low affinity for ORs [65,66] and minimal analgesic or respiratory depressant activity [65,67]. However, M3G is not inert since 1) intracerebroventricular [68,69] or intrathecal [70,71] injections of M3G cause behavioral excitation, 2) rats develop hyperaesthesia and allodynia after intrathecal M3G [70], and 3) intracerebroventricular injections of M3G elicit electroencephalographic spiking, excessive grooming, and epiliptoform discharges [68,72]. Taken together, the latent effects of morphine on the ventilatory responses to hypoxia may be due to the actions of M3G in the brain and/or periphery via mechanisms other than the activation of ORs.
5. CONCLUSION
The ventilatory responses during hypoxic challenge were depressed in MOR rats even though its effects on ABG chemistry, A-a gradient and Vm had fully subsided before challenge. Whether these latent effects are due to morphine or a metabolite is yet to be determined. Opioid-induced hypoventilation, upper airway obstruction, and destabilization of breathing during sleep are major concerns in humans [73,74]. Adults with obstructive sleep apnea (OSA) are at risk for respiratory complications to opioids given post-operatively [73]. Moreover, children undergoing adenotonsillectomy for relief of OSA symptoms have a higher sensitivity to the ventilatory depressant effects of μ-OR agonists, and higher post-operative difficulty in breathing compared to those without OSA [73,74]. There may be many patients with undiagnosed OSA [75], which are at risk for impaired breathing post-operatively [76-78]. The present study suggests that even normal patients may be under threat from opioids that blunt ventilatory responses to hypoxia even when monitoring suggests that the effects of opioids on ABG chemistry and Vm had subsided. Considering the ability of opioids to destabilize breathing during sleep, the latent deleterious actions of opioids on ventilatory responses to hypoxia may be especially dangerous when the patient is asleep. Our studies suggest that M6G in humans and M3G in rats should be further investigated with respect to their potential ability (and mechanisms of action) to exert deleterious effects on the hypoxic ventilatory response.
6. ACKNOWLEDGEMENTS
This work was supported by grants from Galleon Pharmaceuticals (S. J. L).
NOTES
#Corresponding author.